Heat dissipation in electronic devices is becoming increasingly important because the increasing packaging density of these devices results in a large amount of heat being generated in a compact packaging space, and poor heat dissipation can result in a significant loss of longevity and performance stability in electronic products. In order to improve heat dissipation, electrical insulating materials with high thermal conductivity are required. A common approach to improving the heat dissipation capability of electronic products is to use polymer matrix composites containing thermally conductive fillers.

Typically, silicon dioxide, alumina, aluminum nitride, boron nitride, etc. are widely used as insulating thermally conductive fillers. Silicon dioxide is cost-effective, but its thermal conductivity is low, and its heat dissipation ability cannot cope with the increase in heat generation. Alumina has a higher thermal conductivity than silicon dioxide, and thus better heat dissipation, but the disadvantage is that its hardness is high, and the manufacturing equipment is prone to wear and tear. Aluminum nitride, boron nitride and other nitride-based fillers have excellent thermal conductivity, but are expensive and have limited applications. Magnesium oxide thermal conductivity than silicon dioxide is one order of magnitude higher, about twice as much as aluminum oxide [45-60W/(m.K), reference value from Konoshima], hardness is lower than alumina (alumina Moh’s hardness 9, magnesium oxide Moh’s hardness 6), which can reduce the wear and tear of manufacturing equipment, and its price is lower than the nitride series of thermally conductive filler, is regarded as ” Thermally conductive fillers” are considered to be excellent candidates. In Ref. 1, researchers achieved thermal conductivities of up to 3 W/(m.K) for epoxy molding compounds (EMCs) using MgO fillers with a volume fraction of 56 vol%. The thermal conductivity of EMCs obtained with 56 vol% filler is approximately twice as high as that of conventional silica-filled EMCs with the same volume fraction of filler, and has equivalent electrical insulating, thermal expansion, and water-absorption properties.
In Reference 1, researchers used MgO fillers with a volume fraction of 56% to achieve thermal conductivities of up to 3 W/(m.K) for epoxy molding compounds (EMCs).The thermal conductivity of EMCs obtained with 56 vol% filler was approximately twice that of conventional silica-filled EMCs with the same volume fraction of filler, and had equivalent electrical insulating, thermal expansion, and water absorption properties.
However, one of the fatal injuries of magnesium oxide is that it is more hygroscopic than silica and alumina, and when it hydrates with atmospheric moisture, the volume expansion that occurs can lead to problems such as cracks and decreased thermal conductivity in the composites, and so there is a need to improve the hydrolysis resistance of the magnesium oxide in order to enhance its usefulness. In addition, when using magnesium oxide as a thermally conductive filler, it is necessary to have a high filler in the resin composites to obtain higher heat dissipation performance, so high mobility and good compatibility with the matrix magnesium oxide is also and its importance. The moisture resistance of magnesium oxide can be effectively improved by special surface treatment.
In Reference 2, researchers conducted a hydrothermal reaction under CO2 pressurization to obtain core-shell structure powders of magnesium carbonate (MgCO3) coated magnesium oxide with excellent moisture resistance. Magnesium carbonate is a stable compound with low solubility in water, and the nucleus of the magnesium oxide particles should be completely covered by the shell of the magnesium carbonate reaction layer in order to avoid the reaction between magnesium oxide and water, however, the thermal conductivity of magnesium carbonate is 15 W/(m-K), which is lower than that of MgO, and the production conditions should be controlled so that the shell (magnesium carbonate) is as thin as possible and sufficiently dense to ensure that water will not pass through the shell. Details of the process can be found in reference 2. The moisture-resistant magnesium oxide powder described in Japanese Tecai Show 6H83648 is manufactured in a multi-step process in which, first, the magnesium oxide powder is fired. Thereafter, a silica film is formed on the magnesium oxide powder by spray plating, chemical deposition or spray bonding to coat the surface of the magnesium oxide powder. Alternatively, the surface of the magnesium oxide powder is coated with a silica film by mixing micronized silica in the magnesium oxide powder and firing it. Because of this solution, there is a problem of increasing the manufacturing process and the need for equipment for manufacturing.
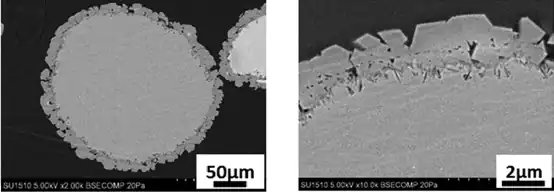
The moisture-resistant magnesium oxide powder described in Japanese Tecai Zhao 6H83648 is manufactured in a multi-step process in which, first, the magnesium oxide powder is sintered. Thereafter, a silica film is formed on the magnesium oxide powder by spray plating, chemical deposition or spray bonding to be coated on the surface of the magnesium oxide powder. Alternatively, the surface of the magnesium oxide powder is coated with a silica film by mixing micronized silica in the magnesium oxide powder and firing it. Because of such a solution, there is a problem of increasing the manufacturing process and the need for equipment for manufacturing.
In patent document CN102485804A, a low hygroscopicity magnesium oxide powder was manufactured by a simple method and applied to a thermosetting resin composition. The electrically insulating layer made with the resin composition has excellent moisture resistance, excellent processability, and good thermal conductivity, and is suitable for use as an insulating layer for circuit boards such as printed circuit boards on which heat-generating parts are mounted. The inventors used magnesium oxide with a silica mass content of 1 to 6% as a raw material and fired it at 1650°C to 1800°C (a temperature near the melting point of silica). By this operation, the silica exuded on the surface of the magnesium oxide powder does not completely separate from the magnesium oxide powder, but is coated on the surface of the magnesium oxide powder, forming a silica film on the surface of the magnesium oxide powder. There is no need for a special process, but only the original firing process of the magnesium oxide powder can be used, which can simplify the manufacturing process, and the surface of the magnesium oxide powder is coated with a silica film, which can improve the hygroscopicity of the magnesium oxide powder. According to the patent document CN102485804A, if the silica content is less than 1% of the total mass, the molten silica cannot sufficiently cover the surface of the magnesium oxide powder, and the improvement of reducing the hygroscopicity of the magnesium oxide powder cannot be accomplished. In addition, if the silica content is more than 6% of the total mass, the thickness of the silica film covering the surface of the magnesium oxide powder becomes thicker, the thermal conductivity of the magnesium oxide cannot be utilized, and the thermal conductivity of the resin molding material is reduced.
In order to improve the filling rate of the powder to the resin, it is necessary to make the shape of the powder close to spherical, and the patent document CN1839182A mentions a spherical coated magnesium oxide powder whose surface is coated by the composite oxides, and the composite oxides coated on the surface of the magnesium oxide powder preferably contain one or more elements selected from aluminum, iron, silicon and titanium with magnesium, such as magnesium olivine (Mg2SiO4), spinel ( Al2MgO4), magnesium ferrite (Fe2MgO4), magnesium titanate (MgTiO3) and the like. The first purpose of the composite oxide coating is to improve the moisture resistance of the magnesium oxide powder, and the second purpose is to make the processing step of spheronization of the magnesium oxide powder easy. Spheronization is made easy by forming a composite oxide with a lower melting point than the flame temperature on the surface of the magnesium oxide powder, so that the surface of the magnesium oxide powder has a low melting point. The melting point of the composite oxide is preferably 2773 K or less thereof, and more preferably 2273 K or less thereof. In addition, it is mentioned in the invention document that the spherical magnesium oxide powder can be surface treated with a silane-based coupling agent, a titanate-based coupling agent, and an aluminate-based coupling agent as needed, which can further improve the fillability. Silane coupling agent: vinyl trichlorosilane, vinyl trialkoxysilane, epoxypropoxypropyltrialkoxysilane, methacryloyloxypropylmethyldialkoxysilane, and so on. Titanate-based coupling agent: isopropyl triisostearyl titanate, tetraoctyl bis(bis-tridecyl phosphate) titanate, bis(dioctyl pyrophosphate) oxyacetate titanate, and the like.
CN1930084A provides a method for manufacturing a phosphorus-containing coated magnesium oxide powder having good water resistance, and a resin composition containing the powder. Composite oxide-coated magnesium oxide has good water resistance, but is still prone to areas of incomplete coating. In this invention, in order to improve the water resistance by filling the incomplete coating area of the compound oxide on the surface of the magnesium oxide powder, a magnesium phosphate compound is further formed on the coating layer formed by the compound oxide, and a magnesium oxide powder with excellent water resistance is obtained.
