The effect of different pyrolysis conditions on the appearance of magnesium carbonate crystals is mainly reflected in the change of crystal morphology.
The effect of pyrolysis temperature on the morphology of magnesium carbonate crystals
- 50℃: rod-shaped magnesium carbonate with length of 36-88μm and diameter of 2μm was prepared.
- 60℃: flakes and rose petal-shaped magnesium carbonate composed of flakes were prepared.
- 70℃: spherical magnesium carbonate with a diameter of about 40μm and a smooth surface was prepared.
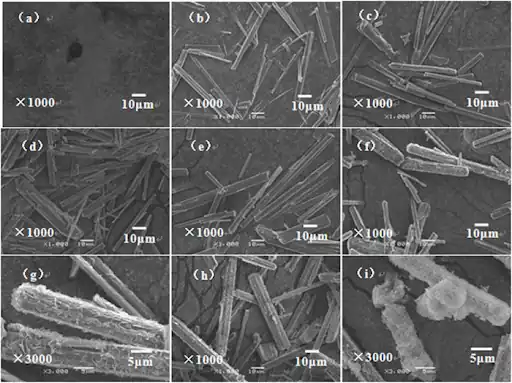
Effect of additives on the morphology of magnesium carbonate crystals
- Sodium citrate: the addition of sodium citrate at a pyrolysis temperature of 50 ℃ can make the rod-shaped basic magnesium carbonate particles distributed uniformly.
- Ethanol: at a pyrolysis temperature of 50 ℃, the addition of ethanol can reduce the length-to-diameter ratio of rod-shaped basic magnesium carbonate.
- Magnesium chloride: adding magnesium chloride at a pyrolysis temperature of 50 ℃ can make the length of rod-shaped basic magnesium carbonate crystals become about 115 μm and the diameter of about 18 μm.
Effect of pyrolysis reaction rate on the morphology of magnesium carbonate crystals
Reducing the partial pressure of CO2 gas and increasing the rate of pyrolysis reaction will slow down the transformation process of light magnesium carbonate crystal morphology from rod → flake → sphere. When the pyrolysis temperature is 50℃, increasing the reaction rate will promote the agglomeration of light magnesium carbonate crystals, and the faster the reaction rate, the more obvious the agglomeration phenomenon.
In summary, the pyrolysis temperature, additives and pyrolysis reaction rate can significantly affect the morphology of magnesium carbonate crystals, and different morphologies of magnesium carbonate crystals can be prepared by adjusting these conditions.

