In this experiment, fibrous alkaline magnesium chloride (Mg2(OH)3Cl-4H2O) was prepared from MgCl2-6H2O with ammonia as raw material and methanol-water as reaction solvent, and the yield was more than twice as much as that when water was used as solvent. The utilization rate of magnesium was further improved by recycling the filtrate. Fibrous alkaline magnesium chloride (Mg2(OH)3Cl-4H2O) was used as raw material and reacted with sodium hydroxide in ethanol-water system to prepare fibrous magnesium hydroxide, and surface modification was carried out by using surfactants to finally obtain fibrous activated magnesium hydroxide with high surface activity and relatively large length diameter. Fibrous basic magnesium chloride was prepared from MgCl2-6H2O with ammonia in methanol-water system.
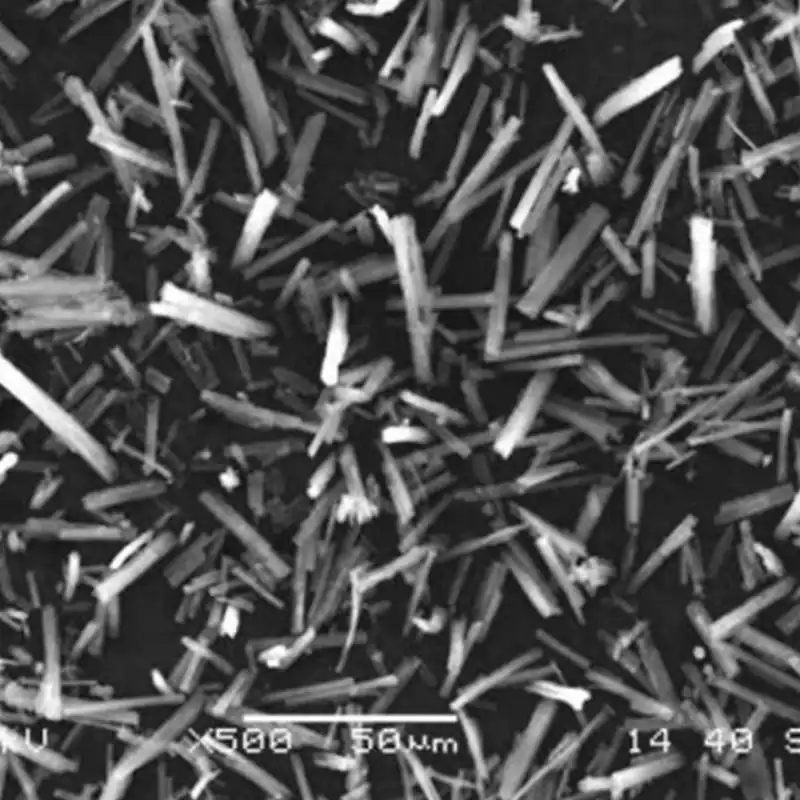
The influences of methanol concentration, molar ratio of MgCl2 to NH3-H2O, MgCl2 concentration, reaction temperature, and aging temperature on the yield and morphology of basic magnesium chloride were investigated and optimized by orthogonal experiments. The optimal preparation process was obtained as follows: methanol concentration of 25.0%, MgCl2 to NH3-H2O molar ratio of 3.0:1, MgCl2 concentration of 4 mol-L-1, reaction temperature of 25 °C, and aging temperature of 50 °C. The yield of 13.13% was obtained. Fibrous alkaline magnesium chloride with a yield of 13.13%, a length of 30-50 μm, a diameter of about 0.3 μm, an aspect ratio of more than 100 and a molecular formula of Mg2(OH)3Cl-4H2O was obtained. Fibrous basic magnesium chloride (Mg2(OH)3Cl-4H2O) and sodium hydroxide were used as raw materials for the preparation of fibrous magnesium hydroxide in an ethanol-water system.
The effects of material molar ratio, ethanol concentration, reaction temperature, and concentration of basic magnesium chloride slurry on the reaction time and the morphology of magnesium hydroxide were investigated. The results of orthogonal experiments showed that when the molar ratio of sodium hydroxide and basic magnesium chloride was 2.0:1, the solvent was 80% ethanol, the reaction temperature was 50 ℃, and the concentration of basic magnesium chloride was 0.30 mol-L-1, the fibrous magnesium hydroxide with a smooth surface, a length of 30-50 μm, a diameter of about 0.3 μm, and an aspect ratio of more than 100 could be obtained in only 1.5h. The conversion rate was 100%.
In the process of surface modification of fibrous magnesium hydroxide, the effects of modifier type, modifier dosage, modification temperature, magnesium hydroxide slurry concentration, modification time and other factors on its modification effect were investigated, and a more suitable modifier, sodium stearate, was selected to carry out surface modification. The experimental results showed that when the dosage of sodium stearate was 5%, the modification temperature was 50°C, the concentration of magnesium hydroxide slurry was 2%, and the modification time was 1.5h, the produced fibrous magnesium hydroxide had an activation degree of 99.12%, a length of 30-50 μm, a diameter of about 0.3 μm, and an aspect ratio of more than 100.
