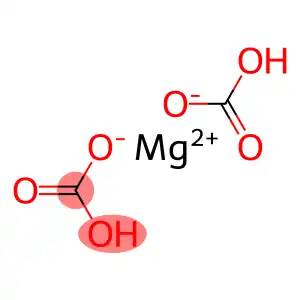
Magnesium bicarbonate is an ionic compound consisting of a magnesium cation (Mg2+) and two bicarbonate anions (HCO−3). It has a chemical formula of Mg(HCO3)2. It is a relatively unstable compound and, due to its higher energy levels, can quickly decompose into magnesium carbonate (MgCO3), carbon dioxide (CO2) and water (H2O) under normal conditions.
It has been studied for its potential health benefits in the area of heart health and prevention of heart disease by reducing risk. However, more research is needed to fully comprehend the extent of its usability for health purposes in humans.
Magnesium Bicarbonate
Magnesium bicarbonate is a white, crystalline powder and is soluble in water. It is believed to have several health benefits as magnesium is an essential mineral responsible for a variety of bodily functions such as muscle and nerve function, blood pressure regulation and energy production.
Bicarbonate is an important buffer that maintains the pH balance of the body. It is obtained by treating magnesite, dolomite, dolomitic limestone and other such minerals containing magnesium carbonate with water containing free carbon dioxide. This compound exists only in solution form. It has the ability to cause temporary hardness when it enters water. Its IUPAC nomenclature gives it the name magnesium hydrogen carbonate.
Magnesium Bicarbonate Properties
Some main physical and chemical properties of magnesium bicarbonate are as follows.
Physical Properties
| Physical Properties | |
| Taste | Slightly bitter |
| Appearance | Exists only in liquid form |
| Odour | Odourless |
| Solubility | Extremely soluble in water to 5.7g/100 ml at 20°C |
| Monoisotopic mass | 145.97 g/mol |
| Charge | +2 |
| Chemical formula | Mg(HCO3)2 |
| Molar mass/ Molecular weight | 146.3387 |
| pH | 8.3 |
| Boiling point | 333.6°C at 760 mmHg |
Chemical Properties
- Magnesium bicarbonate being an extremely unstable compound, easily decomposes into magnesium carbonate, free carbon dioxide gas and water.
Mg(HCO3)2→MgCO3+H2O+CO2
- Magnesium bicarbonate is highly soluble in water and can form complex ions in solution.
Preparation of Magnesium Bicarbonate
Magnesium bicarbonate cannot be prepared directly as it is not a stable compound. However, it can be produced by a reaction of magnesium hydroxide with water and carbon dioxide, which is known as the magnesium bicarbonate water treatment process. Here are the steps of this synthesis:
- Treating magnesium acetate with sodium bicarbonate.
Mg(CH3COO)2+2NaHCO3→Mg(HCO3)2+2CH3COONa
- Magnesium hydroxide, also called milk of magnesia, and pressurized carbon dioxide, which is seltzer water, react to form the magnesium bicarbonate solution.
Mg(OH)2+2CO2→Mg(HCO3)2
- When this solution is dried and decomposed, it converts magnesium bicarbonate to magnesium carbonate and releases water and carbon dioxide as its by-products.
Mg+2HCO3→MgCO3+CO2+H2O
Magnesium Bicarbonate: Hardness of Water
Hard water is water which has a high amount of minerals in it. Magnesium bicarbonate is one of the chemicals that causes this hardness. Magnesium bicarbonate is an extremely unstable compound and so, when it is mixed with water, it immediately decomposes into magnesium carbonate, water and carbon dioxide under normal conditions. Hence, it causes temporary hardness in water.
Magnesium Bicarbonate Uses
Some of its main uses are as follows.
- Magnesium bicarbonate is used for the medical treatment of hyperacidity induced medical conditions such as peptic ulcer, gastritis, hiatal hernia and reflux esophagitis since it acts as an antacid.
- It acts as a pH buffer in the human body.
- It is used in the management of renal insufficiency.
- High concentrations of magnesium bicarbonate are necessary in the body in order to excrete calcium from soft tissues.
- It is employed as an acidity regulator and additive in the food industry.
- It is used in a variety of industries as an alkali, bleaching agent and anti-cracking substance.


