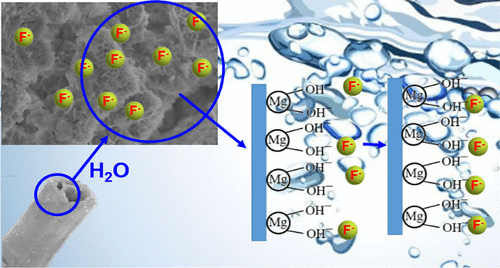
The essence of the reaction mechanism for fluoride removal using magnesium oxide is the “slow dissolution of Mg²⁺, combining with F⁻ to form MgF₂ precipitate, accompanied by a certain degree of surface adsorption.”
1. Basic Reaction Equations
In aqueous solution, magnesium oxide (MgO) reacts with fluoride ions (F⁻) to form magnesium fluoride (MgF₂) precipitate:
MgO + 2HF → MgF₂↓ + H₂O
or simplified in aqueous solution as:
Mg²⁺ + 2F⁻ → MgF₂↓
Magnesium oxide hydrolyzes in water to form magnesium hydroxide, which further releases Mg²⁺, reacting with F⁻ to form a precipitate:
MgO + H₂O → Mg(OH)₂ → Mg²⁺ + 2OH⁻
2. Analysis of Reaction Process Mechanism
① Magnesium Oxide Hydrolysis and Mg²⁺ Release:
Magnesium oxide itself is a basic oxide, insoluble in water but capable of partial hydrolysis, slowly releasing Mg²⁺ ions. This “slow-release” characteristic makes the reaction relatively mild and controllable.
② Fluoride Ion Binding with Mg²⁺:
F⁻ possesses strong nucleophilicity and readily combines with Mg²⁺ to form magnesium fluoride, which has very low solubility and precipitates quickly, thus reducing the fluoride concentration in the water.
③ Coexistence of Adsorption and Precipitation:
Some fluoride ions may initially be adsorbed onto the surface of magnesium oxide or magnesium hydroxide before being converted into magnesium fluoride precipitate. This indicates that the process involves:
- Surface adsorption (physical/chemical adsorption)
- Ionic reaction in solution (precipitation)
3. Key Factors Influencing Mechanism Performance
- pH Value: Magnesium oxide exhibits the strongest reactivity in neutral to weakly alkaline conditions; the reaction is limited at excessively high pH.
- Temperature: Increasing the temperature can accelerate both the hydrolysis and precipitation processes, but it should not be too high to avoid affecting the material’s structure.
- Fluoride Concentration: At high fluoride concentrations, magnesium fluoride forms rapidly, leading to a faster fluoride removal rate; at low concentrations, the process becomes more reliant on adsorption.
- Stirring Speed: Affects the degree of contact between the particle surface and ions in the solution.
- Magnesium Oxide Crystal Form and Specific Surface Area: Nano-sized magnesium oxide or porous structured materials are more conducive to improving the reaction rate and adsorption capacity.
4. Research Methods for Mechanism Study
- XRD (X-ray Diffraction): Detects the crystal structures of magnesium oxide and the formed magnesium fluoride.
- FTIR (Fourier Transform Infrared Spectroscopy): Analyzes changes in surface functional groups.
- SEM/TEM (Scanning Electron Microscopy/Transmission Electron Microscopy): Observes the surface morphology of particles and the structure of the precipitate.
- ICP-MS (Inductively Coupled Plasma Mass Spectrometry): Monitors the concentration changes of F⁻ and Mg²⁺ in the solution.
- Adsorption Isotherm Fitting: Helps understand whether physical adsorption or chemical reaction is the dominant mechanism.
