Abstract
For the first time, surface modifiers such as stearic acid, zinc stearate, or rare earth coupling agents were introduced into a traditional azeotropic distillation system, allowing the surface modification and drying processes of $Mg(OH)_2$ nanoparticles to be completed simultaneously4. The structure, morphology, and properties of the samples were characterized using XRD, TEM, FT-IR, TG, and CAM (contact angle measurement) techniques5. The results show that the surface modifiers not only chemically react with the $OH^-$ ions on the surface of the $Mg(OH)_2$ nanoparticles to form a surface modification layer—thereby reducing hard agglomeration—but also change the nanoparticle surface from hydrophilic to hydrophobic6. Additionally, the mechanism of preparing surface-modified nanoparticles via azeotropic distillation is discussed, providing a new approach for the surface modification of nanoparticles7.
Keywords: Azeotropic distillation, surface modification, magnesium hydroxide, preparation8.
1. Introduction
To solve the problem of nanoparticle agglomeration, surface modification is often performed, which has been a research hotspot in the field of nanomaterials9. While liquid-phase preparation of nanoparticles offers simple synthesis processes and low costs, the large amount of hydroxyl groups and water molecules between nanoparticles often causes them to agglomerate and grow, forming large hard agglomerates10. Therefore, adding surfactants, coupling agents, or polymer monomers directly into the liquid phase reaction system can generate a modification layer on the surface while the nanoparticles are forming11. The resulting steric hindrance increases nanoparticle stability and changes surface properties, such as shifting from hydrophilic to lipophilic12.
Additionally, controlling the drying method can reduce or eliminate hard agglomeration caused by capillary and hydrogen bonding actions13. Methods include supercritical drying, freeze-drying, and azeotropic distillation drying14. However, supercritical and freeze-drying require high temperature or pressure and harsh conditions15. In contrast, azeotropic distillation is simple and effective. In this process, hydroxide precipitates form a uniform suspension with n-butanol; when heated to $93^{\circ}C$, water content in the azeotropic vapor reaches 44.5%. As distillation proceeds, water is removed until the boiling point of n-butanol ($117^{\circ}C$) is reached, effectively dehydrating the oxides and preventing hard agglomeration16.
Magnesium hydroxide ($Mg(OH)_2$) is a green, environmentally friendly inorganic flame retardant17. This paper introduces surface modifiers (stearic acid, zinc stearate, rare earth coupling agents) into the azeotropic distillation drying system for the first time, successfully achieving surface modification of $Mg(OH)_2$ nanoparticles simultaneously with drying18.
2. Experimental Part
2.1 Reagents and Instruments
- Reagents: Magnesium chloride hexahydrate ($MgCl_2 \cdot 6H_2O$), Ammonia water ($NH_3 \cdot H_2O$), n-Butanol ($C_4H_9OH$), Stearic acid ($C_{17}H_{35}COOH$), Zinc stearate ($(C_{17}H_{35}COO)_2Zn$)19. All reagents were analytical grade. The rare earth coupling agent (WOT) was a commercial product20.
- Instruments: X’Pert Philips XRD diffractometer, JEM-2010EX/S Transmission Electron Microscope (TEM), AVATAR 360 FT-IR system, EXSTAR 6000 Thermal Analysis System, and Dropmaster 300 Liquid/Solid Interface Analyzer21212121.
2.2 Synthesis of Surface Modified $Mg(OH)_2$ Nanocrystals
First, 10g of $MgCl_2 \cdot 6H_2O$ was dissolved in 50mL of water. At a specific temperature, 2 mol/L $NH_3 \cdot H_2O$ was slowly added at a rate of 2 mL/min until pH reached 10, with rapid stirring22. After the reaction, the product was washed with distilled water to remove impurity ions. The resulting filter cake was ultrasonically dispersed into a pre-prepared n-butanol solution (containing the different surface modifiers)23.
The uniform slurry was transferred to a round-bottom flask for azeotropic distillation. When the temperature rose to $93^{\circ}C$ (water/n-butanol azeotropic temperature), all water and some n-butanol were distilled off. As the temperature rose to $117^{\circ}C$ (boiling point of n-butanol), the remaining n-butanol was distilled off, yielding surface-modified $Mg(OH)_2$ nanocrystals24. To remove free, physically adsorbed modifiers, the samples were extracted with anhydrous ethanol in a Soxhlet extractor for 4 hours and finally dried in an oven at $90^{\circ}C$ for 6 hours25.
3. Results and Discussion
3.1 XRD Analysis
The X-ray powder diffraction patterns (Fig. 1) of pure $Mg(OH)_2$ nanoparticles ($Mg(OH)_2$ NPs), stearic acid modified particles ($Mg(OH)_2$-St), zinc stearate modified particles ($Mg(OH)_2$-ZnSt), and rare earth coupling agent modified particles ($Mg(OH)_2$-WOT) show no obvious differences 26. This indicates that the reaction between the modifiers and $Mg(OH)_2$ occurs only on the surface, forming a thin modification layer without affecting the bulk structure27. All samples correspond to the hexagonal $Mg(OH)_2$ lattice constants (JCPDS 7-239) and exhibit good crystallinity28. The average grain size calculated by the Scherrer formula is 15.8 nm29.

a.Mg(OH)2; b.Mg(0H)2-St; c.Mg(OH)2-ZnSt; d. Mg( OH)2-WOT
3.2 TEM Analysis
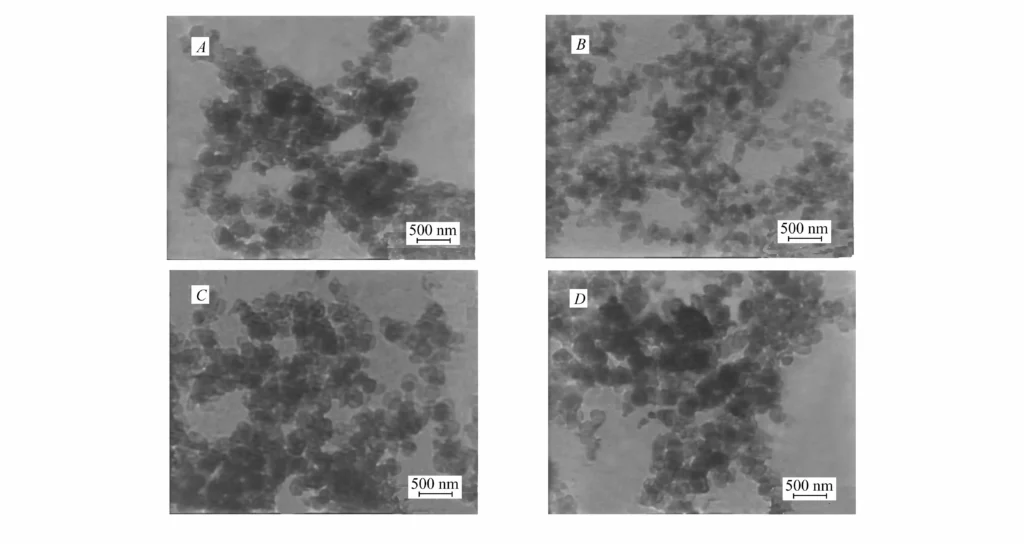
TEM images (Fig. 2) show that the $Mg(OH)_2$ nanoparticles have a plate-like morphology but uneven size30. The modifiers did not significantly affect the morphology, confirming surface modification occurs after particle formation. However, the steric hindrance from the modifiers significantly improved dispersion in chloroform, particularly for $Mg(OH)_2$-St and $Mg(OH)_2$-ZnSt, whereas unmodified $Mg(OH)_2$ could not be dispersed in chloroform even with ultrasound31.
3.3 FT-IR Analysis
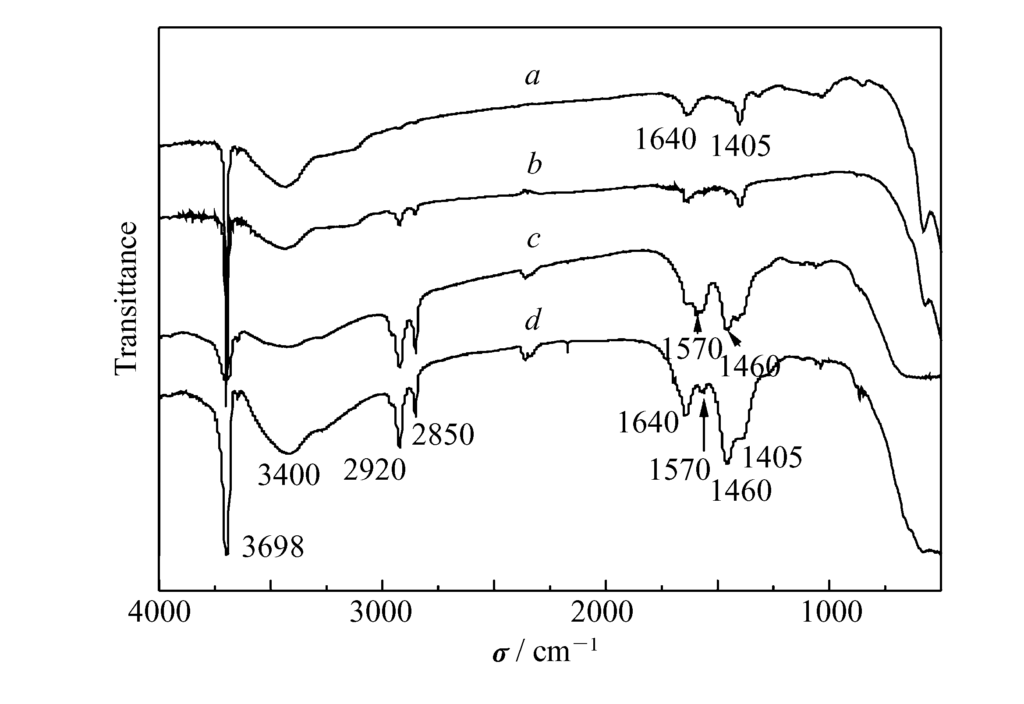
FT-IR spectra (Fig. 3) reveal a strong absorption peak at $3698 cm^{-1}$ for all samples, characteristic of the -OH bond in the $Mg(OH)_2$ crystal structure32. The modified samples (curves b, c, d) show absorption peaks at 2850 and $2920 cm^{-1}$ (C-H stretching vibration), indicating aliphatic carbon chains are attached to the $Mg(OH)_2$33. Peaks at 1570 and $1460 cm^{-1}$ in curves b and c correspond to the bidentate symmetric and asymmetric stretching of magnesium stearate, proving that stearic acid or zinc stearate chemically reacted with $Mg^{2+}$ ions on the surface34. The rare earth coupling agent likely forms a stable Re-O bond on the surface35.
3.4 TG Analysis
The TG curves (Fig. 4) show that pure $Mg(OH)_2$ loses weight between $315-380^{\circ}C$ due to decomposition into MgO and $H_2O$36.
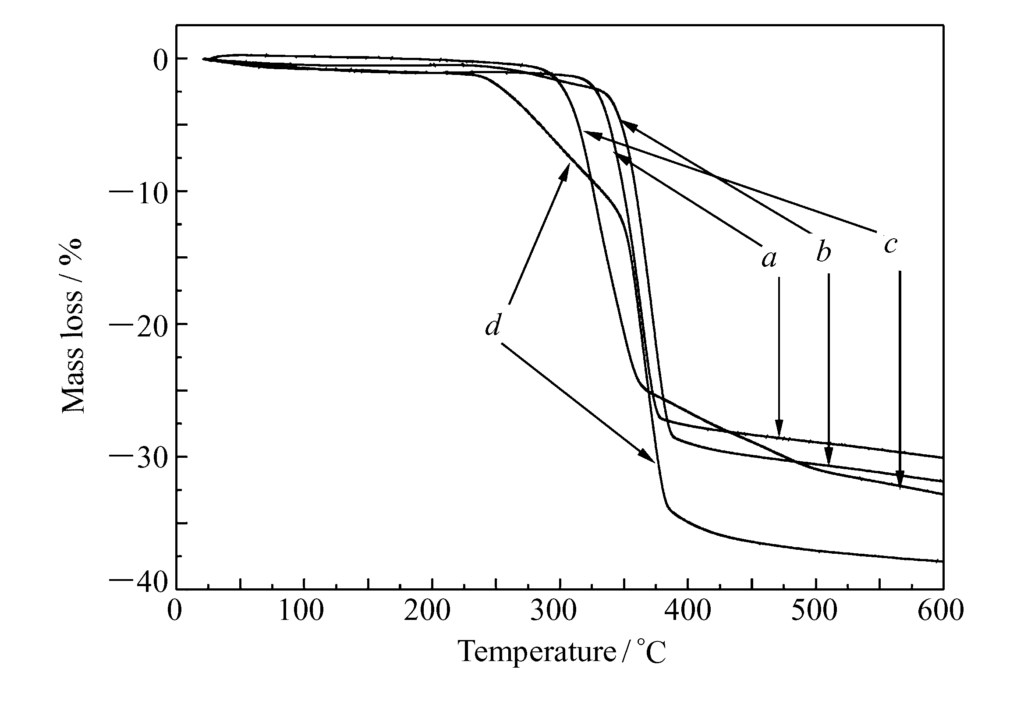
- For $Mg(OH)_2$-St, weight loss begins earlier at $250^{\circ}C$ due to the thermal oxidative decomposition of the surface stearic acid37.
- For $Mg(OH)_2$-ZnSt, the decomposition temperature shifts to lower temperatures ($285-365^{\circ}C$)38.
- For $Mg(OH)_2$-WOT, decomposition shifts significantly lower, beginning at $230^{\circ}C$39.
The weight loss for all modified samples is greater than that of the bulk $Mg(OH)_2$ due to the decomposition of the organic coating40.
3.5 Hydrophobic Performance Analysis
Contact angle (CA) measurements (Fig. 5) demonstrate the change in surface properties:
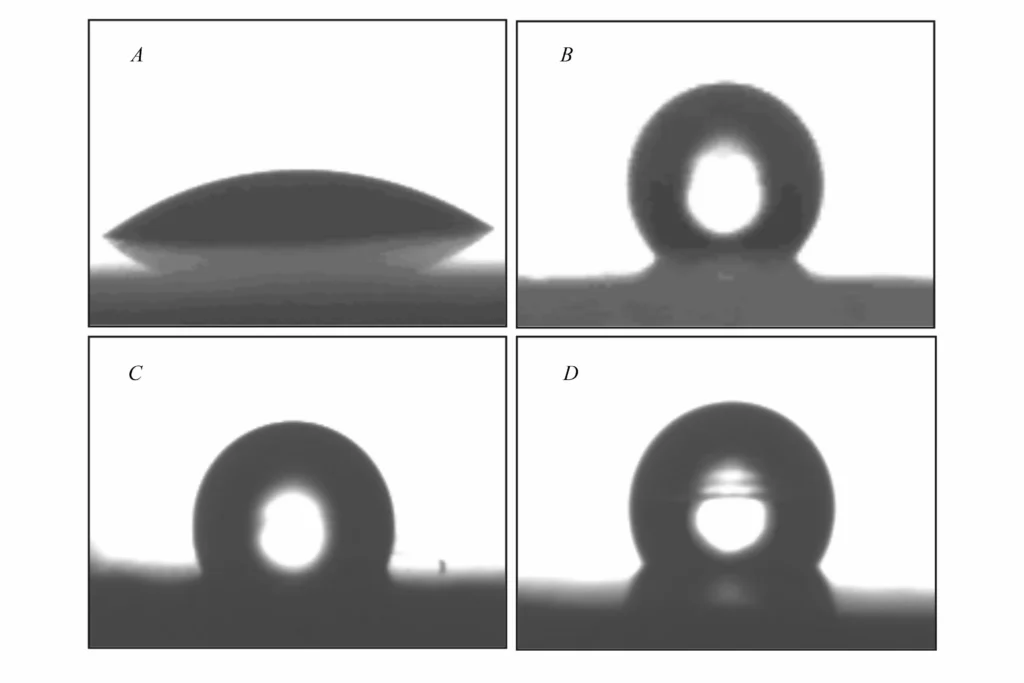
- Unmodified $Mg(OH)_2$ (A): Shows rapid wetting with a contact angle of only about 30° (hydrophilic)41.
- Modified Samples: Contact angles increased significantly to greater than 90° (hydrophobic).
- $Mg(OH)_2$-St (B): Largest contact angle, approx. 135°42.
- $Mg(OH)_2$-ZnSt (C): Approx. 100°43.
- $Mg(OH)_2$-WOT (D): Approx. 120°44.This transition from hydrophilic to hydrophobic allows better dispersion in polymer matrices45.
3.6 Mechanism of Azeotropic Distillation Surface Modification
The mechanism is illustrated in Fig. 6. In the $Mg(OH)_2$ filter cake, surface $OH^-$ ions form hydrogen bonds with water46. When mixed with n-butanol and a modifier:
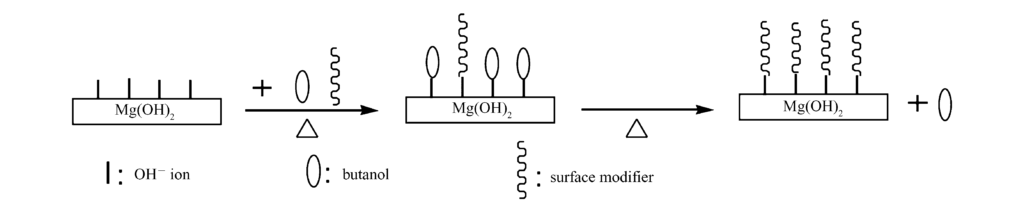
- As temperature rises, n-butanol and modifiers replace water molecules via hydrogen bonding with surface $OH^-$47.
- At the azeotropic temperature ($93^{\circ}C$), water is distilled out. Since the filter cake has little water and n-butanol is in excess, water is completely removed48.
- As temperature rises to the boiling point of n-butanol ($117^{\circ}C$), the n-butanol is distilled off, and the modifiers (initially hydrogen-bonded) chemically react with the surface to form a permanent modification layer49.
Chemical Reactions:
- Stearic Acid: $Mg(OH)_2 + 2C_{17}H_{35}COOH \longrightarrow (C_{17}H_{35}COO)_2Mg + 2H_2O$50.
- Zinc Stearate: $Mg(OH)_2 + (C_{17}H_{35}COO)_2Zn \longrightarrow (C_{17}H_{35}COO)_2Mg + ZnO + H_2O$51.
4. Conclusion
The study successfully introduced surface modifiers (stearic acid, zinc stearate, rare earth coupling agent) into an n-butanol azeotropic distillation system for the first time52. This allowed for the simultaneous drying and surface modification of $Mg(OH)_2$ nanoparticles. The process changed the particle surface from hydrophilic to hydrophobic through chemical bonding53. The method is simple, highly operable, and provides a novel approach for nanoparticle surface modification with potential for wider application54.
