Currently, aluminum hydroxide is widely used in China. However, as the processing temperatures of polymers continue to rise, aluminum hydroxide tends to decompose easily, which reduces its flame-retardant effectiveness. Consequently, magnesium hydroxide is gradually replacing aluminum hydroxide. Compared to aluminum hydroxide, magnesium hydroxide offers the following advantages:
- Higher Thermal Stability: The decomposition temperature of magnesium hydroxide is 330°C, which is 100°C higher than that of aluminum hydroxide. This allows for higher plastic processing temperatures, faster extrusion speeds, and shorter molding cycles.
- High Decomposition Energy: Magnesium hydroxide has a high decomposition energy, which helps absorb the heat of combustion and improves flame-retardant efficiency.
- Strong Acid Neutralization: It possesses a strong capacity to neutralize acidic gases such as CO₂, SO₂, and NOx produced during the combustion of plastics.
- Equipment Protection and Smoke Suppression: With low hardness and superior smoke suppression capabilities, magnesium hydroxide causes less friction on machinery, thereby extending the service life of production equipment.
When magnesium hydroxide is heated to temperatures between 340°C and 490°C, it decomposes and absorbs heat from the surface of the combustible material, providing a flame-retardant effect. Simultaneously, it releases a large amount of water vapor to dilute the oxygen concentration at the combustion surface. The resulting active magnesium oxide residue adheres to the surface of the combustible material, further preventing the combustion process.

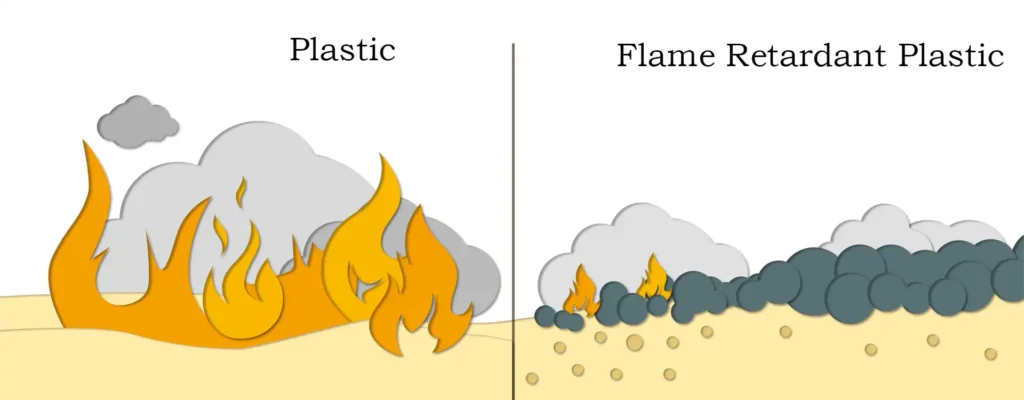
Furthermore, magnesium hydroxide does not produce any harmful substances throughout the flame-retardant process. Its decomposition products not only provide flame retardancy but also absorb significant amounts of smoke and harmful gases generated by the combustion of high polymers like plastics and rubber. The active magnesium oxide continuously absorbs incompletely burned molten residues, quickly halting combustion while eliminating smoke and preventing melt dripping. It is a rising eco-friendly inorganic flame retardant.
Flame retardants can be classified into two major categories based on their chemical composition: organic flame retardants and inorganic flame retardants. Organic flame retardants are further divided into halogen-based and phosphorus-based series. Due to drawbacks such as heavy smoke and high toxicity of decomposition products, organic flame retardants are gradually being replaced by inorganic alternatives.
The primary types of inorganic flame retardants include magnesium hydroxide, aluminum hydroxide, tin oxide, antimony oxide, molybdenum oxide, red phosphorus, zinc borate, and ammonium molybdate. Among these, magnesium hydroxide and aluminum hydroxide are particularly effective because of their high heat absorption during decomposition and the production of water vapor, which isolates the material from air. Additionally, the oxides formed after decomposition are high-temperature resistant substances. Therefore, these two flame retardants serve a dual purpose as both flame retardants and fillers. They are characterized by being non-corrosive (no halogen gases), non-toxic, smoke-free, non-dripping, non-volatile, and having long-lasting effects.
