Physical barrier: Magnesium carbonate particles can form a dense chalk layer on the surface of the material, blocking the contact between oxygen and combustible materials, reducing the burning rate.
Heat absorption: Magnesium carbonate will decompose into magnesium oxide and carbon dioxide when heated, absorbing a large amount of heat, lowering the surface temperature of the material and delaying the occurrence of combustion.
Release of non-flammable gas: Carbon dioxide produced by the decomposition of magnesium carbonate is a kind of non-flammable gas, which can dilute the concentration of oxygen in the combustion area and inhibit the combustion.
Promote carbonization: Magnesium carbonate can promote the formation of carbon layer on the surface of the material, which has good heat insulation and flame retardant properties.
In addition, magnesium carbonate has the following advantages:
Non-toxic, odorless, non-corrosive, harmless to human body and environment.
Low price and abundant source.
Easy to disperse and process.
Application examples of magnesium carbonate

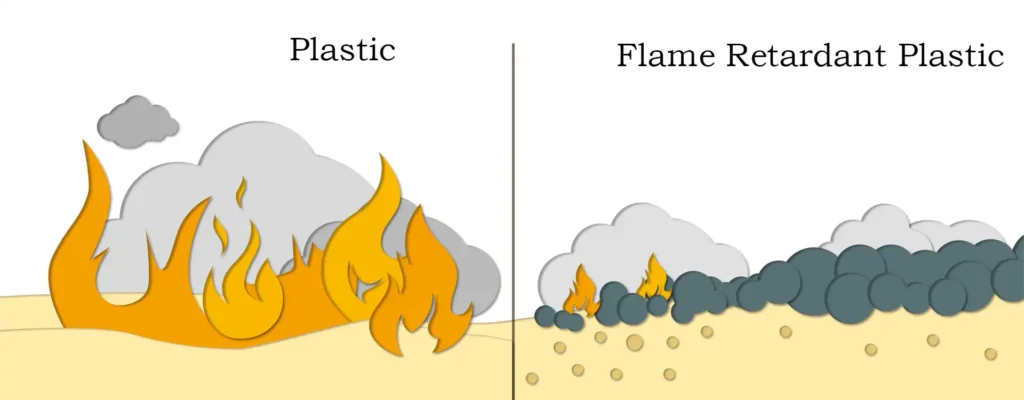
Plastics: Magnesium carbonate is widely used in PVC, PE, PP and other plastic products of flame retardant and smoke suppression, can significantly improve the fire performance of the material.
Rubber: Magnesium carbonate can be used for flame retardant and smoke suppression of rubber products, such as tires, conveyor belts, cable sheathing and so on.
Textiles: Magnesium carbonate can be used for flame retardant and anticorrosion of textiles, such as clothing, home textiles, industrial protective equipment.
Others: Magnesium carbonate can also be used for flame retardant and smoke suppression in paint, coating, paper making and other industries.
It should be noted that the flame retardant effect of magnesium carbonate will be affected by its particle size, addition amount and dispersion state. In order to get the best flame retardant effect, we need to choose the right magnesium carbonate product and adding method according to the specific application.

