According to Messi Biology, magnesium gluconate is an organic salt containing magnesium, commonly used to supplement the human body’s required magnesium element. It has high bioavailability and low gastrointestinal irritation. Magnesium oxide plays the role of providing magnesium ions in the production process of magnesium gluconate.
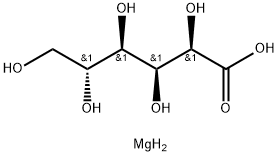
In the manufacturing process, magnesium oxide can be used as a magnesium source to react with gluconic acid, generating magnesium gluconate. The specific chemical reaction process may involve dissolving magnesium oxide in an appropriate amount of acid (such as dilute hydrochloric acid or sulfuric acid) to produce a soluble magnesium salt. This solution is then reacted with gluconic acid or its precursor to form a magnesium gluconate precipitate. The product is obtained after steps such as filtration, washing, and drying.
Magnesium oxide mainly plays the following roles in this process:
- Magnesium Source: Magnesium oxide is a high-purity magnesium source, ensuring that the final product contains sufficient magnesium.
- Reactant: It is one of the important reactants in the synthesis of magnesium gluconate, chemically bonded to the gluconic acid molecule through a chemical reaction to form a stable compound.
- pH Control: Because magnesium oxide is an alkaline substance, it can be used in some cases to adjust the pH of the reaction system, thereby optimizing reaction conditions and improving product yield and purity.
Messi Biology is a high-tech enterprise focusing on the research and development and production of pharmaceutical and food-grade magnesium oxide. Pharmaceutical-grade magnesium oxide is mainly used in the pharmaceutical industry as a pharmaceutical excipient or active ingredient. The products meet strict national and international standards, such as the Chinese Pharmacopoeia (ChP), the United States Pharmacopeia (USP), or the European Pharmacopoeia (Ph. Eur.), which can ensure their purity, safety, and effectiveness.
