Hebei Messi Biology Co., Ltd. states that when you see a bottle of magnesium L-threonate supplement, it is highly likely prepared through carefully controlled chemical reactions between magnesium oxide and L-threonic acid. This choice represents the optimal solution, resulting from a combination of chemical engineering, cost control, and product quality requirements. Magnesium oxide (MgO) is the most commonly used and crucial starting raw material (magnesium source) for the industrialized production of Magnesium L-threonate. Its role is not just to provide magnesium ions; its chemical properties are particularly well-suited for this specific synthesis process.
The Core Role of Magnesium Oxide as a Magnesium Source
The synthesis of magnesium L-threonate is a salt formation reaction. This process requires:
- An alkaline substance providing magnesium ions: Magnesium oxide (MgO)
- An acidic substance providing the acid radical: L-Threonic Acid
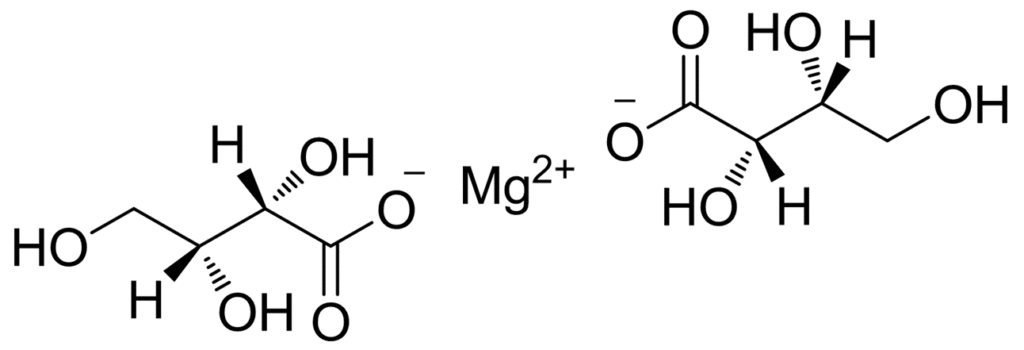
Magnesium oxide acts as a “base” here, undergoing an acid-base neutralization reaction with L-threonic acid to form magnesium L-threonate salt and water.
The chemical reaction equation can generally be expressed as:
2HOOC-CH(OH)-CH(OH)-CH₂OH (L-Threonic Acid) + MgO → (HOOC-CH(OH)-CH(OH)-CH₂O)₂Mg (Magnesium L-Threonate) + H₂O
Why Choose Magnesium Oxide Over Other Magnesium Compounds?
- High Magnesium Content: The mass percentage of magnesium in magnesium oxide is as high as 60%. This means that less magnesium oxide raw material is needed to produce a unit weight of magnesium L-threonate, leading to high efficiency and lower costs.
- Mild Reaction, Easy to Control: Compared to highly reactive calcium oxide (CaO) or strongly corrosive magnesium hydroxide (Mg(OH)₂), MgO has lower solubility in water. This makes the reaction process milder and easier to control the reaction rate and temperature, avoiding localized overheating or the formation of byproducts.
- Fewer Impurities, High Purity: High-purity pharmaceutical-grade or food-grade magnesium oxide is readily available. The only reaction byproduct is water, with no other ions (such as chloride ions Cl⁻, sulfate ions SO₄²⁻) remaining. This ensures the high purity of the final product. If magnesium chloride (MgCl₂) or magnesium sulfate (MgSO₄) were used, it would introduce difficult-to-remove chloride or sulfate impurities.
- Environmentally Friendly: The entire reaction exhibits high “atom economy,” producing almost no waste, which aligns with the principles of green chemistry.
Application in the Specific Preparation Process
- Raw Material Preparation: High-purity magnesium oxide powder is mixed with L-threonic acid (which usually exists in its lactone form and requires prior hydrolysis) in precise stoichiometric ratios.
- Reaction Process: The stirred reaction is conducted in a specific solvent (typically water) at a controlled temperature. Magnesium oxide slowly dissolves and reacts with L-threonic acid.
- pH Adjustment: The amount of magnesium oxide added is crucial and needs to be precisely controlled to ensure complete reaction and to bring the reaction system’s pH value to the most stable range for magnesium L-threonate.
- Purification and Crystallization: After the reaction is complete, the solution is concentrated, cooled for crystallization, or spray-dried to obtain pure magnesium L-threonate powder.
- Quality Control: The final product is tested to ensure its chemical purity, heavy metal content, moisture content, and other indicators comply with pharmaceutical or food-grade standards.
Throughout the entire process, the particle size, reactivity (calcination degree), and purity of magnesium oxide are critical parameters affecting the reaction rate and the quality of the final product.
