Magnesium oxide (MgO) is an important catalyst support or co-catalyst in methane reforming reactions. Due to its high thermal stability, strong surface basicity, and good resistance to carbon deposition, it is widely used in various methane conversion reaction systems, such as:
- Steam Reforming (SRM)
- Dry Reforming (DRM)
- Partial Oxidation (POM)
- Tri-reforming
The goal of these reactions is to react CH₄ with H₂O, CO₂, or O₂, etc., to produce syngas (H₂ + CO), which is used to synthesize hydrogen, methanol, Fischer-Tropsch fuels, and more.
1. Mechanism of Action of Magnesium Oxide
In methane reforming, magnesium oxide is usually used as a support or forms composite catalysts with other metal oxides (such as Ni, Co, La, Ce, etc.), playing the following roles:
(1) Surface Basicity Regulation:
- The basicity of the magnesium oxide surface helps to adsorb and activate CO₂, promoting the DRM reaction.
- It reduces the tendency for carbon deposition (coke) and improves the catalyst lifespan.
(2) Provision of Structural Stability:
- The high melting point of magnesium oxide (approximately 2852℃) ensures that the catalyst does not undergo structural collapse at high reforming reaction temperatures (700–900℃).
- It forms strong metal-support interactions (SMSI) with metals, enhancing metal dispersion.
(3) Inhibition of Metal Sintering:
- It prevents the agglomeration of active metals such as Ni or Co at high temperatures, maintaining the high activity of the catalyst.
2. Application of Magnesium Oxide in Different Methane Reforming Reactions
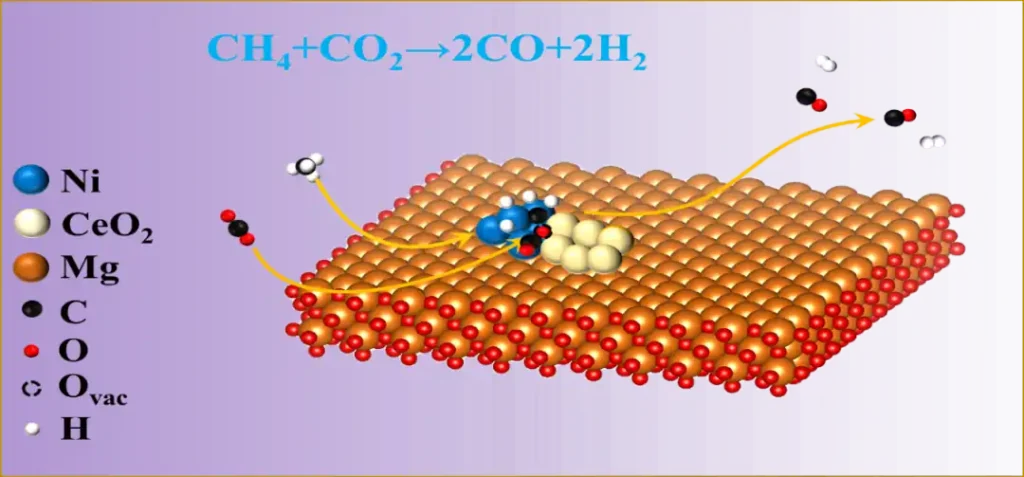
(1) Dry Reforming of Methane (DRM)
- Reaction Equation:
CH₄ + CO₂ → 2H₂ + 2CO (ΔH = +247 kJ/mol) - Characteristics: Endothermic reaction, usually carried out at 700–900℃, can simultaneously utilize two greenhouse gases (CH₄ + CO₂).
- Advantages of Magnesium Oxide:
- Inhibition of coke formation: The basic surface can adsorb CO₂, participating in carbon removal (C + CO₂ → 2CO).
- Forms Ni-Mg-O solid solutions when combined with Ni, improving resistance to carbon deposition and thermal stability.
- When synergistic with oxides such as CeO₂ and ZrO₂, it can enhance oxygen mobility and anti-sintering properties.
- Examples:
- Ni/MgO catalysts exhibit high stability and resistance to carbon deposition.
- Ni–MgO–La₂O₃ ternary composite systems improve the H₂/CO ratio and catalytic lifespan.
(2) Steam Reforming of Methane (SRM)
- Reaction Equation:
CH₄ + H₂O → CO + 3H₂ (ΔH = +206 kJ/mol) - Role of Magnesium Oxide:
- Acts as a basic support to regulate the activity of the metal and the equilibrium of the water-gas shift reaction (CO + H₂O → CO₂ + H₂).
- Avoids metal sintering or catalyst poisoning caused by excessive acidity.
- Examples:
- Magnesium oxide can be used together with Al₂O₃ as a composite support for Ni (Ni/MgO-Al₂O₃), exhibiting strong stability.
- Ni-MgO catalysts show good selectivity at high H₂ yields and can tolerate partial sulfur poisoning.
(3) Partial Oxidation of Methane (POM)
- Reaction Equation:
CH₄ + ½O₂ → CO + 2H₂ (ΔH = -38 kJ/mol) - Characteristics:
- Exothermic reaction, often used for rapid reaction applications.
- The syngas ratio (H₂/CO) is close to 2, suitable for Fischer-Tropsch synthesis.
- Contribution of Magnesium Oxide:
- Improves the redox ability of metal oxides such as Ni.
- Helps to inhibit complete oxidation (CH₄ → CO₂ + H₂O), increasing the selectivity for CO and H₂.
- Enhances oxygen transfer capability and improves catalytic efficiency when composited with ZrO₂.
(4) Tri-reforming
- Overall Reaction Equation (Comprehensive):
CH₄ + CO₂ + H₂O + O₂ → syngas (H₂ + CO) - Characteristics:
- Combines DRM, SRM, and POM to improve thermodynamics and reaction rates.
- Better control over the syngas composition (H₂/CO) to meet downstream requirements.
- Role of Magnesium Oxide:
- Participates in the basic activation of multiple reaction pathways.
- Maintains a stable structure, especially during the vigorous reactions involving oxygen.
- Used in combination with CeO₂, La₂O₃, ZrO₂, etc., to improve oxygen vacancy concentration and redox performance.
