Magnesium oxide, also known as magnesia, is a magnesium oxide with a chemical formula of MgO. Its molecular structure is a typical NaCl structure, with magnesium atoms and oxygen atoms arranged in order. Magnesium oxide is a highly functional fine inorganic material that can be widely used in catalysis, electronics, ceramics and other fields. Magnesium oxide has a large specific surface area and is a promising catalyst carrier that can be used in a variety of reactions. It is also a promising chemical adsorbent that can destructively adsorb various pollutants. In addition to the above-mentioned applications, due to its characteristics, magnesium oxide can also be used to make effect pigments and sensing materials under certain conditions [1].
1 Preparation of magnesium oxide with special morphology
There is often a strong correlation between the microscopic morphology and properties of materials, so the morphology control of inorganic materials has been one of the hot topics in the field of materials research in recent years. In recent years, there have been reports at home and abroad about magnesium oxide with special morphology and nanostructure. Many experimental studies have found that magnesium oxide with special morphology has very effective applications in many aspects. The following is a brief introduction to some special morphology magnesium oxide. The preparation is summarized.
1.1 Spherical magnesium oxide
Magnesium oxide with a spherical structure is calcined from the precursor spherical basic magnesium carbonate or spherical magnesium hydroxide or spherical basic magnesium oxalate. Taking spherical basic magnesium carbonate as an example, the spherical structure of basic magnesium carbonate is formed by stacking basic magnesium carbonate with a flaky structure. Basic magnesium carbonate is calcined, and the spherical basic magnesium carbonate is thermally decomposed to obtain spherical magnesium oxide [2].
There are two main technical routes for preparing spherical magnesium oxide: (1) using magnesium salt as raw material to first obtain a precursor for preparing spherical magnesium oxide, and heat-treating the precursor to obtain spherical magnesium oxide; (2) mixing magnesium oxide powder with a solvent and a binder After mixing the mixture (in some cases, no binder is needed), the spherical magnesium oxide is obtained through mechanical molding, and then the spherical magnesium oxide product is obtained through heat treatment.
Zhang[3] et al. used a precipitation method to obtain spherical magnesium oxide with a diameter of 15-17um. They continued to study the effect of time on its reaction process and found that during the synthesis process, magnesium oxide first became flake-shaped, and then gradually became filamentous. Finally it becomes spherical [3].
1.2 Tubular magnesium oxide
The tubular magnesium oxide reported in the current literature is generally in the nanometer range in diameter and micron in length. It usually has the characteristics of directional growth, good crystallinity and definite crystal plane orientation. Magnesium oxide nanotubes can be prepared using the carbon-thermal evaporation method. During preparation, an appropriate amount of Ga2O3 is added to the mixture of magnesium oxide and carbon. Ga2O3 plays a vital role in the formation process of magnesium oxide nanotubes. At high temperatures, Carbon reduces Ga2O3 to obtain gallium vapor, and the condensed gallium droplets catalyze the anisotropic growth of magnesium oxide nanotubes in situ. The obtained magnesium oxide nanotubes are single crystals with an average outer diameter of 200nm, a wall thickness of 20nm, and a length of up to 50um [4].
1.3 Flake magnesium oxide
The key to obtaining flaky magnesium oxide by ultrasonic treatment lies in the impact of the exploded bubbles on the layered magnesium oxide during the ultrasonic cavitation phenomenon, causing the layered magnesium oxide to peel off. Ultrasonic waves will cause the liquid molecules to be continuously compressed and stretched, creating alternating positive and negative pressure areas, causing the water molecules to alternate between dense and dense. Microbubbles will be obtained in the sparse areas and grow up in the negative pressure areas. When the microbubble bursts, the surrounding water phase will quickly rush into the center of the bubble, releasing a strong pressure pulse. The pressure of this pulse is often higher than 104KPa. This phenomenon is also called the ultrasonic cavitation effect.
This method uses the mechanism of ultrasonic cavitation, using ultrasonic waves to cause the continuous expansion and compression of the liquid phase to generate a large number of microbubbles, and cause the microbubbles to continuously generate shock waves in the positive pressure area to hit the surface of the material, making a layer of layered magnesium oxide One layer is peeled away, creating magnesium oxide nanosheets.
1.4 Magnesium oxide whiskers
Magnesium oxide whiskers are mainly prepared by the precursor calcination method, that is, precursor whiskers such as basic magnesium sulfate, basic magnesium chloride, basic magnesium carbonate, and magnesium carbonate are first prepared, and then the magnesium oxide whiskers are obtained by heat treatment.
Using activated magnesium oxide and magnesium chloride as raw materials, basic magnesium chloride whiskers are first synthesized under hydrothermal conditions. The morphology of basic magnesium chloride whiskers can be maintained after pyrolysis, thereby obtaining magnesium oxide whiskers. The length of the whiskers is about 200um and the diameter is about 0.5um.
Using magnesium sulfate and sodium hydroxide as raw materials, the precursor basic magnesium sulfate with good crystallization and fibrous shape was prepared through hydrothermal crystallization at room temperature. By controlling the decomposition rate of the precursor to slowly decompose at low temperatures to maintain the whisker-like shape, and then sintering at high temperatures, magnesium oxide whiskers that are well sintered, evenly dispersed, and have a large aspect ratio can be obtained [5].
1.5 Mesoporous magnesium oxide
Mesoporous magnesium oxide is mainly prepared by using mesoporous carbon, cotton fiber, etc. as hard templates. Control the water bath temperature of a solution containing 1mol·L-1Mg(NO3)2 with a volume of 200mL at 30°C, and quickly add it to it under rapid stirring. The temperature is room temperature, the volume is 200mL, and the concentration is 0.8mol·L-1 K2CO3 solution (stoichiometric ratio). At this time, the solution changed from clear to liquid-solid mixed state, and the stirring state was maintained for 30 minutes. The suspension was separated from liquid and solid, and soluble ions were removed with distilled water. The sample prepared in this experiment had good filterability. The resulting sample was then heated to 110°C and dried. The dried sample was initially crushed and placed in a The temperature in the alumina crucible was raised to 600°C with the furnace, and the phase transformation time was 2 hours. The obtained sample was rod-shaped mesoporous magnesium oxide [3].
1.6 Preparation and research status of other magnesia with special morphology
Yu et al. used a chemical precipitation method, using magnesium nitrate and potassium carbonate as raw materials, mixing potassium carbonate and magnesium nitrate in a 120°C oil bath environment, continuing to stir for 1 minute, then aging at 120°C for 2 hours, filtering and washing, and then roasting at 700°C. In 4 hours, nest-shaped and flower-shaped magnesium oxide crystals were obtained by changing the ratio of magnesium nitrate and potassium carbonate.
Sutradhar et al. used a hydrothermal method to synthesize flake, rod-shaped, flower-shaped and spherical magnesium oxide using magnesium nitrate and ammonium carbonate as raw materials, and studied its application as a catalyst support.
Wang et al. mixed MgCl2 solution with benzoic acid additive, used sodium hydroxide to adjust the pH of the solution to cause magnesium to precipitate, synthesized a flaky magnesium hydroxide precursor using a hydrothermal method, and replaced the additive with citric acid or ethylenediaminetetraacetic acid. When disodium salt was used, fibrous and discoidal magnesium oxide was obtained [4].
02 Application of special morphology magnesium oxide
Nanostructured magnesium oxide is a material that has received widespread attention in recent years. Compared with traditional magnesium oxide, nanostructured magnesium oxide often has smaller particle size and larger specific surface area, and its growth conditions are deliberately controlled. More specific crystal faces can be exposed, providing a higher density of active adsorption sites. Therefore, it has better optical, electrical, magnetic, thermal and chemical properties than traditional magnesium oxide materials, and is one of the hot spots in scientific research and industrial applications.
2.1 Application of spherical magnesium oxide
Spherical magnesium oxide is mainly used in chromatography stationary phase, adsorption of toxic substances, and as material additives. High performance liquid chromatography (HPLC) is an effective separation technology, and its separation effect is greatly affected by the column packing. The currently used fillers are mainly silica-based fillers. When silica-based fillers are used for separation under alkaline conditions, they have problems such as secondary reactions, long retention times, severe tailing, low efficiency, and poor reproducibility. After research and exploration, it was found that mixing magnesium and aluminum oxides and silica in a certain proportion as the stationary phase of liquid chromatography can solve this problem well, so the application of spherical magnesium oxide in liquid chromatography has become more and more popular. The more attention [6].
In addition, spherical magnesium oxide mesoporous nanosheets exhibit excellent adsorption properties and can adsorb common toxic heavy metal ions and organic pollutants, and are expected to be used in wastewater treatment processes. Others experimented with XRD characterization of spherical magnesium oxide prepared by carbonization and found that the characteristic diffraction peak at 335nm was due to the generation of new energy levels due to induced defects or defect energy levels. From these characteristics, it can be predicted that magnesium oxide microspheres and nanosheets will be a very promising material in plasma display panels or other optical applications.
2.2 Magnesium oxide whiskers are used for material reinforcement
Whiskers are a kind of needle-shaped single crystal material with a diameter of a few tenths to several microns and a length of several microns to hundreds of microns. Due to the complete crystal structure, whiskers have good mechanical strength and are used as plastics, metals, ceramics, etc. Substance modifying additives showing excellent physical and chemical properties and mechanical properties.
The microscopic shape of magnesium oxide whiskers is fibrous, with high melting point, high strength, high elastic modulus, heat resistance, alkali resistance, insulation, thermal conductivity (the thermal conductivity is three times that of alumina), stability and reinforcement It has good toughness. In addition, magnesium oxide whiskers have good resistance to high temperature oxidation. Due to the above-mentioned excellent properties, magnesium oxide whiskers are suitable as reinforcing auxiliary materials for composite materials, especially for the preparation of high-temperature composite materials. They are a new high-tech structural material that has developed rapidly in recent years [7].
2.3 Mesoporous magnesium oxide is used for environmental protection
The negative impact of the greenhouse gas carbon dioxide on the environment is getting more and more attention, and there are more and more studies on carbon dioxide capture. Adsorption is an effective method for capturing carbon dioxide, and alkaline oxides are effective carbon dioxide adsorbents. The binding of magnesium oxide to carbon dioxide is a reversible process. The binding force is stronger than zeolite and weaker than alkali metal oxides. Carbon dioxide is adsorbed by magnesium oxide through physical or chemical effects at room temperature, so magnesium oxide is a more suitable carbon dioxide adsorbent. The adsorption capacity of mesoporous magnesium oxide for carbon dioxide is much higher than that of solid magnesium oxide, and the adsorption capacity increases with the increase of temperature. Mesoporous magnesium oxide can also be used to adsorb and remove fluoride ions from water, and the removal efficiency is much higher than that of ordinary commercial magnesium oxide [8].
2.4 Nano magnesium oxide for antibacterial use
So far, there are few domestic and foreign research reports on the antibacterial properties of magnesium oxide. The proposal of the antibacterial properties of magnesium oxide can be traced back to the mid-1990s. The famous Japanese researcher Sawai used conductivity research to test a series of ceramic powders in order to screen out inorganic materials with better antibacterial properties. Experiments have found that among the 26 common types of ceramic powders, 10 metal oxides and carbides such as CaO, ZnO, and MgO have better antibacterial properties. Among them, MgO has been proven to have strong bactericidal and antibacterial abilities against Gram-positive bacteria, Gram-negative bacteria, and fungi [9].
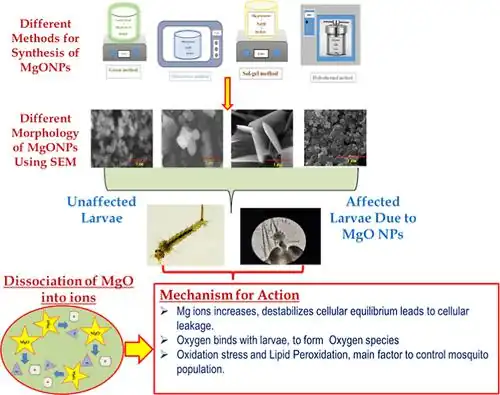
Conclusion
Magnesium oxide series crystal materials, especially magnesium series crystal materials with special morphologies, are widely used in catalysts, insulation materials, antibacterial materials, flame retardants, chemical adsorbents, fine ceramics, etc., while magnesium carbonate and magnesium oxide are used as Magnesium is an important component of the series of crystal materials and also has important application value. In recent years, magnesium oxide has become a research hotspot in the field of materials research. This article focuses on the preparation, application and research status of magnesium oxide with special morphology, and makes a certain discussion and summary from three aspects. It is hoped that it can provide certain references and suggestions for current problems and future research directions.


