Magnesium oxide (MgO) is a widely used chemical material with excellent chemical inertness, heat resistance, insulation, and thermal conductivity. Notably, it possesses good high-temperature oxidation resistance and moderate alkalinity. The presence of oxygen vacancies and unpaired electrons gives it electrophilic properties, providing essential foundational conditions for MgO’s applications.
Generally, magnesium oxide exists as flaky crystals. However, research has found that MgO with specific morphologies has highly effective applications in many aspects. For example, spherical magnesium oxide can be used as a stationary phase material in chromatography, as a material for adsorbing toxic substances, and as an additive in plastics to improve thermal conductivity, among other important and effective applications.
Types of Thermally Conductive Fillers
The thermal conductivity of polymer materials is generally low, with most common materials having a thermal conductivity of around 0.3 W/m·K. Therefore, to improve the thermal performance of polymer materials, thermally conductive fillers need to be added to the polymer matrix. By blending, thermally conductive fillers with higher thermal conductivity are uniformly dispersed in the polymer matrix. The fillers form interconnected thermally conductive networks, so that the polymer material’s thermal performance meets application requirements.
Thermally conductive fillers are mainly divided into three categories: carbon-based materials, metallic materials, and non-metallic inorganic materials. The thermal conductivity of several common fillers is shown in the table below:
| Material Category | Filler | λ (W/m·K) at 25°C | Filler | λ (W/m·K) at 25°C |
| Carbon-Based Materials | Expandable Graphite | 100-400 (In-plane direction) | Pitch-Based Carbon Fiber | 530-1100 (Axial direction) |
| PAN-Based Carbon Fiber | 8-70 (Axial direction) | Carbon Black | 6-174 | |
| Carbon Nanotubes | 2000-6000 (Axial direction) | Diamond | 2000 | |
| Metallic Materials | Al | 204 | Cu | 483 |
| Ag | 417 | Au | 450 | |
| Non-Metallic Inorganic Materials | AlN | 200 | BN | 250-300 |
| Materials | SiC | 270 | Al₂O₃ | 29-30 |
| MgO | 36 | BeO | 260 |
1. Carbon-Based Materials
The thermal conductivity of some carbon-based materials is significantly higher than that of metallic and non-metallic inorganic materials. Carbon-based materials have a unique microstructure, resulting in anisotropic thermal conductivity. Taking graphite as an example, graphite has a typical layered structure and acts through a dual mechanism of electrons and phonons. Therefore, graphite has good thermal conductivity, anisotropic characteristics, is inexpensive, and mixes well with the matrix. It is generally considered the preferred thermally conductive filler.
2. Metallic Materials
Metallic materials are recognized as good conductors of heat and have mature and wide-ranging applications not only as fillers for polymer materials but also in aerospace, machinery manufacturing, and other fields. Metallic materials contain a large number of free electrons, and their thermal conductivity mainly depends on the free movement of these internal electrons. Generally, metallic materials have high thermal conductivity. At the same time, because metallic materials have good electrical conductivity, they can provide electrical conductivity to composite materials prepared as fillers.
However, metallic materials have a high density and are difficult to mix evenly with polymer materials, which limits their application as thermally conductive fillers in polymer materials.
3. Non-Metallic Inorganic Materials
Non-metallic inorganic materials mainly rely on phonon-mediated thermal conductivity, and their thermal conductivity is generally lower than that of carbon-based materials and metallic materials but have better insulation. They are mainly divided into metal nitrides and metal oxides. Metal nitride fillers include BN, AlN, etc.; metal oxide fillers include MgO, Al2O3, etc.
Among them, nitrides exist in the form of crystals, with regular and dense structures, and the resistance of phonons propagating in the crystal is small, so heat can be transferred relatively effectively. However, the higher the purity of the nitride, the higher the price. Although metal oxides do not have high thermal conductivity, they are inexpensive and have a wide range of material sources, so they are widely used.
The most commonly used oxides are alumina and magnesium oxide. Alumina has relatively low thermal conductivity but is inexpensive, so it is widely used. Although the thermal conductivity of magnesium oxide is lower than that of boron nitride, it is higher than that of alumina, at 36 W/m·K, and it is also low in cost. Therefore, it is also receiving increasing attention in thermal conductive filler applications.
Influence of Different Filler Morphologies on Thermal Conductivity
Generally, rod-shaped and flaky structural fillers with a certain aspect ratio are easier to form a thermally conductive network chain when added to polymer materials, thereby improving the thermal conductivity of the composite material. However, such fillers will undergo orientation distribution during the processing, that is, the directions of the rod-shaped structures are inconsistent, which will cause the thermal conductivity of the composite material to be anisotropic. The thermal conductivity in the processing direction is much higher than the thermal conductivity in the direction perpendicular to the processing direction.
Therefore, when designing and producing the shape of filler products, it is necessary to try to make the orientation direction of the fillers consistent, thereby improving the thermal conductivity efficiency of the composite material.
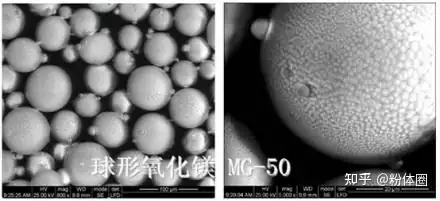
In contrast, due to the isotropy of the spherical structure, spherical fillers have a greater advantage in improving the thermal conductivity of composite materials compared to rod-shaped or flaky structures. At the same time, spherical powder particles have a smaller particle size and are uniformly distributed, the surface morphology is regular, and the packing density of the powder is significantly increased, which can greatly improve the fluidity and dispersibility of the powder, maximize the elimination of agglomeration, and improve the defects inside the powder.
Current Development Status of Spherical Magnesium Oxide
For spherical magnesium oxide products, due to the involvement of high-performance chip technology applications, foreign countries maintain a high degree of confidentiality regarding the sphericalization synthesis technology of magnesium oxide. International procurement of products is difficult, and it is impossible to obtain complete technical parameters and professional manufacturing equipment data. According to relevant literature reports, only a few technologically advanced countries such as Japan, the United States, and Israel currently master the synthesis and manufacturing technology of this product.
Physical and Chemical Methods of Powder Sphericalization
Currently, spherical magnesium oxide is mainly prepared by two methods:
(1) Using magnesium salt as a raw material to first obtain a precursor for the preparation of spherical magnesium oxide, and then heat-treating the precursor to obtain spherical magnesium oxide. Generally, the precursor is spherical basic magnesium carbonate or spherical magnesium hydroxide or spherical basic magnesium oxalate.
(2) The magnesium oxide powder is mixed with a solvent and a binder, and then mechanically formed to obtain spherical magnesium oxide, which is then heat-treated to obtain the spherical magnesium oxide product.
However, in the exploration of industrialization, the sphericalization of magnesium oxide relies more on the technical accumulation based on spherical alumina and spherical silica powder. Globally, Denka is currently at the forefront, and some domestic spherical powder companies are also laying out production lines. It is believed that with the popularity of emerging markets such as 5G and new energy vehicles, spherical magnesium oxide, which is regarded as the “next-generation thermally conductive filler” succeeding spherical alumina, can also be mass-produced as soon as possible, and then begin large-scale application and promotion.
| Category | Physical Method | Chemical Method |
| Principle | Mainly relies on corresponding equipment, using simple mechanical force to interact between powder particles, thereby crushing the material. | Mainly through chemical reactions or phase transitions, where ions, atoms, and molecules undergo crystal nucleus formation and crystal growth to obtain spherical powders. |
| Specific Methods | Mechanical crushing, plasma methods, etc. | Plasma gas-phase method, chemical gas condensation method, precipitation method, emulsion method, sol-gel method, hydrothermal synthesis method, spray pyrolysis method, etc. |
| Characteristics | Low cost, simple process, simple equipment, but large output and high production efficiency. | Commonly used to prepare spherical powders with smaller particle sizes (nanometer scale). |
