High-purity magnesium oxide generally refers to magnesium oxide products with a purity greater than 99%. Due to its excellent physical properties such as ultra-high melting point, good magnetic conductivity and excellent insulation performance, as well as specific chemical properties, high-purity magnesium oxide is widely used in ceramics, metallurgy, medicine, electronics, national defense and other fields.

The development and industrialization of high-purity magnesium oxide will greatly promote the development of industries such as electronics, national defense, aerospace and advanced ceramics. China’s ordinary-grade magnesium oxide products have a large output, are exported in large quantities, and the market is weak; while some fine magnesium oxides such as high-purity magnesium oxide, active magnesium oxide, and silicon steel magnesium oxide have a large market demand, and products are imported in large quantities.
1 Preparation method of ordinary magnesium oxide
The raw materials for producing magnesium oxide mainly come from liquid ores and solid ores. The former is mainly seawater, underground brine, and salt lake brine; solid ores mainly include magnesite, brucite, dolomite, serpentine and asbestos tailings. The main methods for preparing magnesium oxide from brine or bischofite include lime milk method, ammonium carbonate method, ammonia method, soda ash method, and direct pyrolysis method of bischofite; the main methods for preparing magnesium oxide from solid ore include calcined magnesia method, carbonization method, acid hydrolysis method, ammonium sulfate method, and ammonium sulfate double salt method.
2 Preparation method of high-purity magnesium oxide
The production of high-purity magnesium oxide is generally achieved by adopting the preparation method of ordinary magnesium oxide and performing certain purification processing.
2.1 Brine precipitation method
2.1.1 Brine spray thermal decomposition method
After the brine is concentrated to a certain concentration, it is directly sprayed into the thermal decomposition reactor and thermally decomposed at 800℃~1000℃ to produce crude MgO. The crude MgO is washed with water to remove soluble chloride and completely converted into Mg(OH)2, and then calcined at 1600℃~2000℃ to produce high-purity MgO. The process principle is as follows:
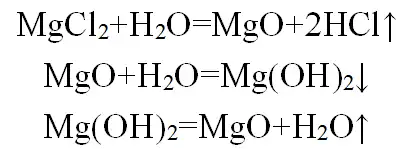
This method uses a secondary calcination process with a high calcination temperature, resulting in high production costs. At the same time, the by-product HCl gas in the primary calcination process is seriously corrosive to the production equipment.
2.1.2 Dolomite/lime method
The dolomite/lime method is a mature and widely used high-purity magnesium oxide production technology. At present, except for a few manufacturers such as Israel’s Dead Sea Periclase (DSP) and Tateho Dead Sea Fused Magnesia (TDF), nearly 20 major high-purity magnesium oxide manufacturers in the world use the dolomite/lime method to produce high-purity magnesium oxide products.
The dolomite/lime method is also called the lime milk precipitation method, which is to add lime milk to brine containing a certain concentration of MgCl2 to react to generate Mg(OH)2, which is then washed, dried, and calcined to obtain MgO. This method has high requirements for the activity of Ca(OH)2. The resulting precipitate is Mg(OH)2, which has small particles and is easy to absorb impurities, so the product purity is low. At the same time, the Mg(OH)2 precipitate is a colloidal substance, which is difficult to filter. The filter cake has a high water content, the drying process consumes a lot of energy, and the generated CaCl2 has a low added value, causing serious environmental pollution.
2.1.3 Ammonia precipitation method
Similar to the dolomite/lime method, the ammonia precipitation method mainly produces magnesium oxide by adding an alkaline precipitant to the brine to prepare a magnesium hydroxide intermediate. The alkaline precipitant used in the ammonia method is liquid ammonia or ammonia gas[10]. The process of producing high-purity magnesium oxide by the ammonia method is as follows: first, the old brine solution is decolorized and refined, and the selected treatment technology is similar to the dolomite/lime method; then, liquid ammonia or ammonia gas precipitant is introduced into the refined old brine solution to react and generate a magnesium hydroxide intermediate; finally, the magnesium hydroxide intermediate is washed, filtered, and calcined to produce a high-purity magnesium oxide product, and the filtrate can be used to produce ammonium chloride as a by-product.
2.1.4 Soda ash method
The soda ash method adds a soda ash solution precipitant to the brine to first generate heavy magnesium carbonate (MgCO3·3H2O) precipitate. The heavy magnesium carbonate is then washed with water, pyrolyzed, and treated to obtain basic magnesium carbonate. Finally, calcination can produce a light high-purity magnesium oxide product. The soda ash method for producing high-purity magnesium oxide is a traditional production method with simple process, low equipment requirements, and high purity of magnesium oxide products. It has a production history of more than 60 years in my country. At present, most medium and small high-purity magnesium oxide production enterprises in my country still use the soda ash method for production.
2.1.5 Ammonium carbonate method
NH4HCO3 is used as a precipitant to react with MgCl2 in the brine to generate 4MgCO3·Mg(OH)2·4H2O. After washing, drying, and calcination, MgO is obtained. The utilization rate of CO2 in NH4HCO3 is low, the production consumes a large amount of NH4HCO3, and the cost is relatively high.
2.2 Solid ore calcination and carbonization method
2.2.1 Magnesite calcination and carbonization method
This method is to calcine magnesite to generate light-burned magnesium oxide, and then digest and carbonize the light-burned magnesium oxide to obtain magnesium bicarbonate solution. Activated carbon is used as an adsorbent to remove iron ions in the magnesium bicarbonate solution. The magnesium bicarbonate solution after adsorption and impurity removal is then pyrolyzed. The basic magnesium carbonate obtained by pyrolysis is filtered, washed, dried, and then calcined to obtain a high-purity magnesium oxide product.
2.2.2 Dolomite calcination and carbonization method
The steps of the dolomite calcination and carbonization method for preparing high-purity magnesium oxide are basically the same as those of the magnesite calcination and carbonization method, which mainly includes five steps: calcination, digestion, carbonization, pyrolysis and calcination.
2.3 Other preparation methods
In addition to the traditional preparation methods described above, some other preparation methods have emerged in recent years, such as microwave radiation, metal alcohol salt hydrolysis, direct precipitation, uniform precipitation, gas phase and sol-gel methods.
Recently, the “galvanic cell method ultra-high purity magnesium oxide” technology independently developed by the Beijing Institute of Technology (Tangshan) Translational Research Center has achieved a breakthrough, solving the technical and industrialization problems that my country has been working on since the 1970s, breaking the situation that this field has been “stuck” by foreign technology. It is understood that in the production process of using magnesium as a fuel cell using the galvanic cell method, it was found that ultra-high purity magnesium oxide can be generated. The galvanic cell method uses magnesium as the anode metal material of the fuel cell, which increases the safety of various links such as fuel cell production, transportation, storage, and filling, and converts magnesium into magnesium oxide in a short time. At the same time, in the process of obtaining ultra-high purity magnesium oxide, no industrial waste discharge can be achieved. Compared with the calcination method of magnesite and the seawater/brine precipitation method, the use of the primary battery process can obtain ultra-high purity magnesium oxide with a purity of up to 99.95%, and has lower costs, shorter process routes, higher product yields, and a large amount of high-quality direct current is generated during the production process.
Summary
With the development of industry, the application scale of high-purity magnesium oxide in traditional fields is expanding, new application fields are also constantly being explored and developed, and the market capacity is constantly expanding. From the development trend, the proportion of high-purity magnesium oxide consumption has increased year by year. However, my country has always lagged behind foreign countries in this field, and has been “stuck” by foreign countries in industrial production technology. Therefore, it is urgent to increase the research and development and industrialization of high-purity magnesium oxide preparation technology.

