Summary
In order to change the surface properties of nano-magnesium hydroxide from hydrophilic to lipophilic, so that it can be better dispersed and filled in polymer materials and reduce agglomeration, a new nano-polyacrylate emulsion is used as a modifier. Surface modification studies were conducted on magnesium hydroxide. The structure and properties of modified nanomagnesium hydroxide were studied by means of activation index test, contact angle test, infrared spectrum analysis and thermogravimetric analysis.
The results show that when the modifier addition amount (mass ratio of modifier to nano-magnesium hydroxide) is 0.6, the surface properties of nano-magnesium hydroxide change from hydrophilic and oleophobic to lipophilic and hydrophobic. The new nano-polyacrylate emulsion It chemically reacts with the surface of nano-magnesium hydroxide particles and coats its surface, achieving a better modification effect. Comparing the modification effect of other traditional silane coupling agents on nano-magnesium hydroxide, the results show that the dispersion and hydrophobicity and lipophilicity of nano-magnesium hydroxide modified by new polyacrylate microemulsion are better than those of traditional silane coupling. Modifying effect of the agent.
Flame retardants refer to additives added to combustible materials that can increase the flame resistance of the material or prevent the material from burning [1]. Adding flame retardants can effectively prevent, delay or terminate the spread of flames when the material is attacked by external fire sources, thereby achieving flame retardant effects [2]. Magnesium hydroxide has the characteristics of flame retardant, smoke elimination, drip resistance, safety, etc. It has good thermal stability, decomposition temperature is as high as 340~490 ℃, and small particle size. It can meet the requirements of mixing and processing of many plastics. It is the most popular magnesium hydroxide in recent years. An environmentally friendly green flame retardant that emerged in 2006 [3,4].
Ordinary magnesium hydroxide has many disadvantages as a flame retardant. Due to its small particle size and high surface energy, nanomagnesium hydroxide is in a thermodynamically unstable state and is prone to aggregation and agglomeration during preparation and application. At the same time, the surface of magnesium hydroxide is hydrophilic and oleophobic, while the polymer material matrix is oleophilic and hydrophobic. The mutual incompatibility of the two will cause the dispersion of magnesium hydroxide in the material to become poor [5, 6, 7]. Uneven dispersion will lead to premature combustion in areas with a small loading of magnesium hydroxide and reduced flame retardant efficiency; in areas where too much magnesium hydroxide is added, it will be difficult for the inorganic particles to be evenly dispersed into the polymer matrix, resulting in Seriously reduce the mechanical properties of polymer materials [8]. Therefore, research on nanonization and modification of magnesium hydroxide has become the key to overcoming these problems [9]. It can reduce the filling amount of flame retardant while improving its compatibility in polymer materials, thereby achieving the improvement of materials. The effect of mechanical properties and flame retardant properties [10].
The traditional modification methods mainly include surfactant method, coupling agent method, and microencapsulation method [11]. According to their stability differences in water, modification methods are divided into dry methods and wet methods [12]. Magnesium hydroxide has poor compatibility in polymers. Surface modification of magnesium hydroxide with different modifiers can improve its dispersion in the polymer matrix and improve the performance of composite materials [13]. The author used a new type of nano-polyacrylate emulsion [14] to conduct surface modification research on nano-magnesium hydroxide, and characterized the structure and properties of the modified nano-magnesium hydroxide so that the modifier could achieve uniform and stable The surface coating changes the surface properties of nano-magnesium hydroxide from hydrophilic and oleophobic to oleophilic and hydrophobic, and can be more uniformly dispersed in polymer materials [15].
1 Experimental part
1.1 Raw materials and instruments
Raw materials: Nano magnesium hydroxide slurry (industrial product); new nano polyacrylate emulsion [made in the laboratory, mass fraction 40%, average particle size 58.6 nm]; deionized water (made in the laboratory); absolute ethanol (chemically pure).
Instruments: JJ-1 mechanical stirrer; TG16-II desktop high-speed centrifuge; 101-1ES electric blast drying oven; VERTEX 70 Fourier transform infrared spectrometer; JC2000C1 static droplet contact angle measuring instrument; STA 449 F3 thermogravimetric analyzer; NOVA 400 field emission scanning electron microscope (SEM).
1.2 Modification experiment
Weigh a certain amount of new nanopolyacrylate emulsion with a mass fraction of 40% and deionized water, pour them into a three-necked flask, and stir and blend at room temperature. Slowly add nano-magnesium hydroxide slurry with a mass fraction of 20% into the three-necked flask through a constant pressure titration funnel, continue stirring at high speed for 4 h, and then transfer to the beaker for aging for 1 h. Centrifuge and wash with deionized water and absolute ethanol respectively, air dry at 80°C for 8 h, and grind to obtain a modified nanometer magnesium hydroxide sample.
1.3 Test characterization
1.3.1 Activation index test
Traditional ordinary nano-magnesium hydroxide is hydrophilic and can naturally settle in water. The modified nano-magnesium hydroxide surface is non-polar, has strong hydrophobicity and large surface tension, allowing it to float on the water surface without sinking. Therefore, the activation index can be used to characterize the modification of nano-magnesium hydroxide. Effect. Activation index test method: Accurately weigh 5.00 g of nano-magnesium hydroxide before and after modification, grind it evenly, place it in a ground-mouth reagent bottle containing 100 mL of deionized water, shake it evenly, let it stand at room temperature for 4 hours, and collect the floating particles. The sample on the water surface was dried and weighed, and the activation index of the sample was calculated. Activation index (H) calculation formula:
H=m1/m2×100%
In the formula: m1 is the mass of the floating part; m2 is the total mass of the sample.
1.3.2 Settlement volume test
The dispersion of modified nano-magnesium hydroxide is enhanced, the agglomeration phenomenon is improved, and the surface property changes to lipophilicity. It can be evenly dispersed in liquid paraffin and sinks very slowly. Therefore, the sedimentation volume can be used to characterize nano-hydroxide. Modifying effect of magnesium. Sedimentation volume test method: Accurately weigh 5.00 g of nano-magnesium hydroxide before and after modification, grind it evenly, place it in a graduated cylinder containing 50 mL of liquid paraffin, stir it evenly, let it stand for a period of time, read and record the sedimentation at different times. The volume of the solid.
1.3.3 Infrared spectrum analysis
Compared with the sample before modification, the modified sample will be coated with a layer of modifier on the surface, so the infrared spectrum of the modified nano-magnesium hydroxide will show certain molecular structures of the modifier. Characteristic absorption peaks of chemical bonds or functional groups. Infrared spectrum test method: Mix and grind the nano-magnesium hydroxide and spectral pure KBr before and after modification at a mass ratio of 1:150, press into tablets, and test with a VERTEX 70 Fourier transform infrared spectrometer. The test wavelength is 500~ 4 000 cm-1, the cumulative number of scans is 28, and the resolution is 4 cm-1.
1.3.4 Contact angle test
The modified nano-magnesium hydroxide is hydrophobic. The contact angle test can most intuitively reflect the hydrophilicity and hydrophobicity of the material. The larger the contact angle, the better the hydrophobicity. Contact angle test method: Use the infrared tableting method to press the nano-magnesium hydroxide powder before and after modification to prepare samples, lay the plates, and then use the JC2000C1 static drop contact angle measuring instrument to test the contact angles respectively.
1.3.5 Thermogravimetric analysis
Thermogravimetric analysis is a technology that accurately measures the relationship between sample mass and temperature change under program-controlled temperature rise. By analyzing the thermogravimetric curve, the composition, thermal stability, thermal decomposition, etc. of the sample can be known. Thermogravimetric test method: The samples before and after modification were tested using the STA 449 F3 thermogravimetric analyzer. The temperature rise rate was 20 ℃/min in a nitrogen atmosphere, and the test temperature range was 30~800 ℃.
1.3.6 Scanning electron microscopy analysis
In order to observe the morphology and size of the samples before and after modification, scanning electron microscopy was used for observation. Scanning electron microscope test method: The samples before and after modification are dried, prepared, sprayed with gold, and then tested using a NOVA 400 field emission scanning electron microscope.
2 Results and discussion
2.1 Comparison of activation index of samples before and after modification
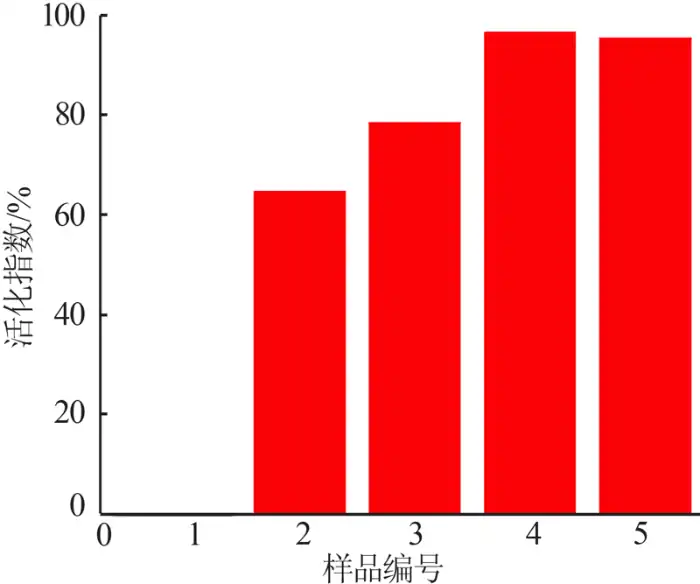
The surface of nano-magnesium hydroxide without surface modification is highly polar and highly hydrophilic. It is easy to mix evenly with water and has a density much greater than that of water, so its floating rate in water is 0; after treatment, The surface of nano-magnesium hydroxide is non-polar and has strong hydrophobicity. The water molecules on the surface have strong surface tension, which makes non-polarity and polarity repel each other, so that the modified nano-magnesium hydroxide powder can Floating on the surface of water; if completely modified, nano-magnesium hydroxide powder will completely float on the water surface, making it completely hydrophobic, so the floating rate is 100%.
Table 1 shows the modified formula of nano magnesium hydroxide. Figure 1 shows the activation index of samples obtained with different amounts of modifier added. The effect of surface modification can be judged based on the activation index. As can be seen from Figure 1: Nano-magnesium hydroxide with a modifier addition amount of 0 can be fully miscible with water due to its hydrophilic and oleophobic properties, so its activation index is 0; modified nano-magnesium hydroxide The surface is non-polar and has a certain degree of hydrophobicity and lipophilicity. At the same time, the activation index first increases and then decreases with the increase in the amount of nano-polyacrylate emulsion added. When the amount of modifier added is 0.6, the activation index of the sample reaches its peak.
| Table 1 Modified formula of nano-magnesium hydroxide | |||
| Sample number | Modifier addition amount | Sample number | Modifier addition amount |
| 1 | 0 | 4 | 0.6 |
| 2 | 0.1 | 5 | 1 |
| 3 | 0.3 | ||
| Note: The amount of modifier added is the mass ratio of modifier to nano magnesium hydroxide. | |||
2.2 Comparison of sedimentation volume of samples before and after modification
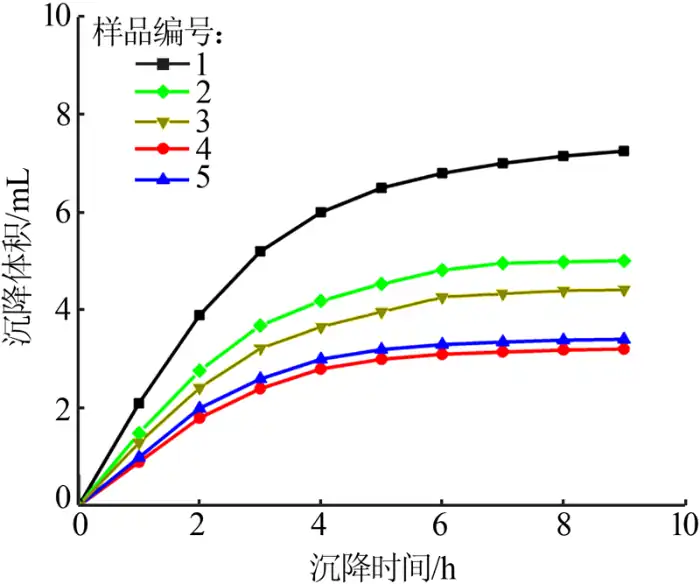
In the liquid paraffin sedimentation experiment, the sedimentation of nano-magnesium hydroxide in liquid paraffin before and after the modification is fast and then slow, and the later sedimentation curve tends to be flat. The modified ideal sample in the liquid paraffin will sink very slowly, because the modified magnesium hydroxide nanoparticles have enhanced dispersion and improved agglomeration phenomenon; on the contrary, the modified undesirable magnesium hydroxide nanoparticles will accelerate the agglomeration due to the role of polarity, and then the sample in the liquid paraffin settling speed is relatively fast.
The size of the settling volume of nano-magnesium hydroxide in liquid paraffin was used to characterize the modification effect. Figure 2 shows the settling volume of the samples in liquid paraffin obtained from different modifier additions. As shown in Figure 2, the nano-magnesium hydroxide with zero modifier addition has polar effect and hydrophilic and hydrophobic characteristics, and it agglomerates seriously in the liquid paraffin, resulting in accelerated sinking and increased sedimentation volume. The surface-modified nano-magnesium hydroxide has lipophilic and hydrophobic properties, which makes it uniformly dispersed in the liquid paraffin and not easy to be agglomerated, so the sedimentation volume is small. The additive amount of the modifier has a slight effect on the settling volume of nano-magnesium hydroxide in liquid paraffin, which shows that the surface property of surface-modified nano-magnesium hydroxide changes from hydrophilic and lipophilic to lipophilic and hydrophobic, so that it can be dispersed in liquid paraffin very well. A better effect can be obtained when the additive amount of the modifier is 0.6, so the following experiments adopt the additive amount of the modifier as 0.6.
2.3 Infrared spectral analysis of the samples before and after modification
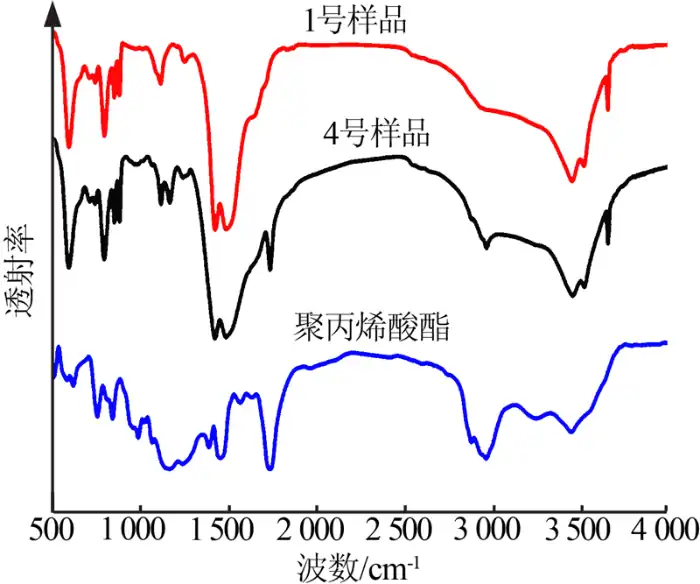
The infrared spectra of the nano-magnesium hydroxide before and after modification were analyzed, and the different characteristic peaks of the samples before and after modification were used to determine whether the modification was successful or not. Figure 3 shows the infrared spectra of the modifier polyacrylate, the nano-magnesium hydroxide without modifier (sample No. 1) and the nano-magnesium hydroxide with modifier (sample No. 4). As can be seen in Fig. 3, the C-H telescopic vibrational absorption peaks of methylene and methyl group appeared at the same time for sample No. 4 (modifier addition of 0.6) and polyacrylate near 2,877 cm-1 and 2,961 cm-1; the carbonyl telescopic vibrational absorption peaks of common ester appeared near 1,724 cm-1; and near 1,375 cm-1 and 1,385 cm-1 the The double peaks near 1,375 cm-1 and 1,385 cm-1 were the stretching vibration of -CH(CH3)2. This indicates that the modified polyacrylate has been adsorbed on the surface of magnesium hydroxide nanoparticles.
2.4 Contact angle test of samples before and after modification
The contact angle test can best visualize the hydrophilicity and hydrophobicity of the material, generally the contact angle less than 60 ° is called hydrophilic contact angle, the contact angle greater than 60 ° is called hydrophobic contact angle. The larger the contact angle, the better the hydrophobicity. Hydrophobic molecules tend to be non-polar and therefore dissolve in neutral and non-polar solutions. Hydrophobic molecules usually clump together in water, while water on the surface of a hydrophobic solution will form a large contact angle and become a droplet. After surface modification, the surface of nano-magnesium hydroxide is nonpolar, so the contact angle test is the most intuitive response to the surface modification effect of nano-magnesium hydroxide test method.
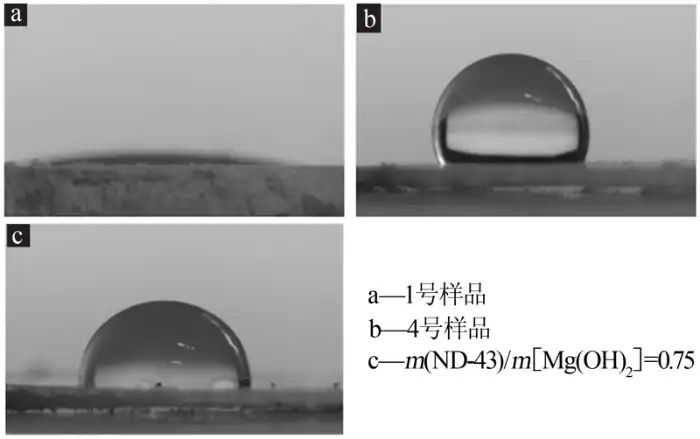
Figure 4 shows the contact angle of nanometer magnesium hydroxide before and after modification. As shown in Fig. 4, when a water drop was pressed on the flat platen of No. 1 sample (modifier addition amount of 0), the water drop was immediately spread on the platen, and the contact angle was measured to be about 0 °, which indicated that the unmodified magnesium hydroxide nanoparticles were very hydrophilic; and the contact angle of the No. 4 sample (modifier addition amount of 0.6) was about 109 °, which was much larger than 60 °, which indicated that the modified magnesium hydroxide nanoparticles had better hydrophobicity and lipophilicity. hydrophobicity and lipophilicity. The optimum contact angle of the silane coupling agent (ND-43) modified sample is about 92°, which is slightly smaller than that of the new nano-polyacrylate emulsion modified sample. It can be seen that the modification effect of the new nano-polyacrylate emulsion on the nano-magnesium hydroxide is superior.
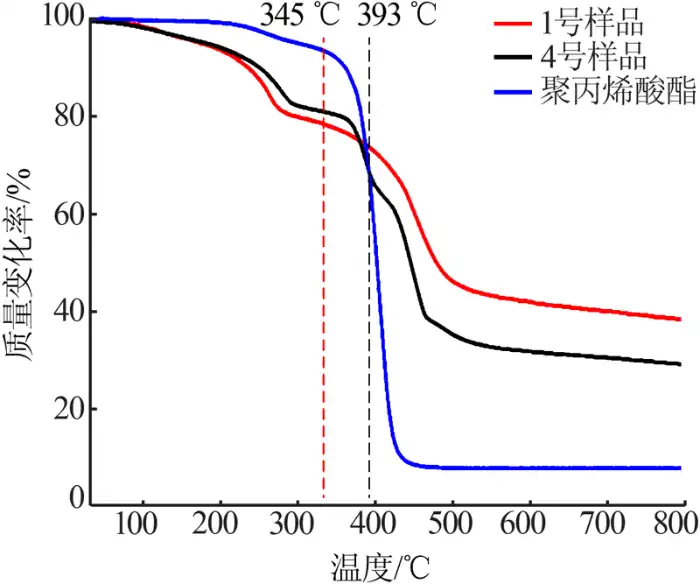
2.5 Thermogravimetric analysis of the samples before and after modification
The thermogravimetric curves of the samples before and after modification were used to characterize the modification effect of the nano-magnesium hydroxide, and the thermogravimetric curves could be analyzed to know the composition of the samples, thermal stability, thermal decomposition and so on. Figure 5 shows the thermogravimetric curves of the samples before and after modification. Figure 5 shows that: the onset thermal decomposition temperature of the modified magnesium hydroxide nanoparticles is about 50 ℃ higher than that of the unmodified magnesium hydroxide nanoparticles; the thermal decomposition curve of the modified sample at about 400 ℃ is almost the same as that of the polyacrylate, and it can be decided that the thermal decomposition of the sample at this time is the thermal decomposition of the polyacrylate adsorbed on the surface of magnesium hydroxide nanoparticles, and the new section of thermal decomposition curve is the thermal decomposition of the polyacrylate after the thermal decomposition of the polyacrylate is complete. After the complete thermal decomposition of polyacrylate, the new thermal decomposition curve is the decomposition curve of magnesium hydroxide, and at the same time, at 799.7 ℃, the residual mass of the sample before modification is 38.31%, and the residual mass of the sample after modification is only 29.11%. This indicates that the novel polyacrylate nanoparticles were successfully adsorbed on the surface of the magnesium hydroxide nanoparticles, which is consistent with the results of infrared spectroscopy.
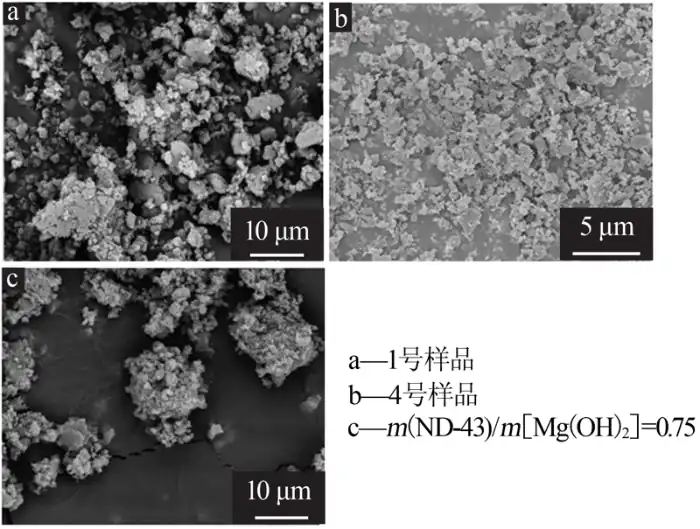
2.6 Scanning electron microscopy analysis of samples before and after modification
The effect of the modifier on the nano-magnesium hydroxide was investigated by analyzing the morphology of the nano-magnesium hydroxide and its size before and after modification by scanning electron microscopy. Figure 6 shows the scanning electron micrographs of the samples before and after modification. By comparing the microscopic morphology of samples No. 1 and No. 4, it can be seen that the coating of a layer of polyacrylate on the surface of magnesium hydroxide nanoparticles reduces the agglomeration phenomenon and improves the dispersion of the samples, indicating that the dispersion of the modified magnesium hydroxide nanoparticles is significantly better than that of the unmodified magnesium hydroxide nanoparticles. At the same time, the size of nanomagnesium hydroxide did not increase due to the adsorption of polyacrylate. Comparing the dispersibility of the samples modified with other modifier (ND-43), it can be seen that the novel nano-polyacrylate emulsion was used for the modification of magnesium hydroxide nanoparticles with better effect.
3 Conclusion
1) The results of activation index characterization showed that the best modification effect was achieved by using the novel nano-polyacrylate emulsion as the modifier with the addition amount of 0.6;
2) The results of infrared spectroscopy showed that the polyacrylates had been adsorbed on the surface of nano-magnesium hydroxide particles through the modification of the novel nano-polyacrylate emulsion;
3) Contact angle test results show that the surface properties of nano-magnesium hydroxide modified by new nano-polyacrylate emulsion change from hydrophilic and oleophobic to lipophilic and hydrophobic, allowing it to be better dispersed in non-polar media;
4) The results of thermogravimetric analysis showed that the modified nano-magnesium hydroxide didn’t affect the thermal stability of its thermal stability, and the onset of its thermal decomposition temperature was higher than that of the unmodified nano-magnesium hydroxide, and the residual mass fraction showed that the modified polyacrylates had already been adsorbed on the surface of the nano-magnesium hydroxide particles, which was consistent with the results of the IR spectral characterization. spectroscopic characterization results.

