Hebei Messi Biology Co., Ltd. stated that there are various methods for preparing nano magnesium oxide. Using magnesium nitrate hexahydrate as a precursor, nano magnesium oxide powder is synthesized by citric acid sol-gel method. Characterization and comparison with magnesium oxide powder prepared by precipitation method show that the sol-gel method has the advantages of high purity, good uniformity, low temperature, easy reaction control, etc., and can be made into various forms such as bulk fiber film powder according to needs.
Nano magnesium oxide material is a new type of high-functional fine inorganic material. It is mainly used as an accelerator and activator for chloroprene rubber, butyl rubber, nitrile rubber and fluororubber. It is a filler for adhesives, plastics, paints and paper. It can also be used to manufacture ceramics, enamel, refractory materials and magnesium oxide cement. It is used as an antacid and laxative in medicine. Active nano magnesium oxide materials have strong adsorption and high surface chemical activity. They can be used as efficient dissociative adsorbents to adsorb toxic chemicals such as chlorinated hydrocarbons, organic phosphides and acidic gases for environmental protection. Nano magnesium oxide film has good high temperature resistance, can be coated on the inner wall of high temperature furnace, and can also be used in the aerospace field; it also has good wear resistance, and has certain moisture-proof and anti-corrosion effects.
1 Experimental purpose and experimental preparation
1.1 Experimental purpose
⑴Preparation of raw materials of nano magnesium oxide and preparation of samples.
⑵Scanning electron microscope phase test and X-ray diffraction analysis of nano magnesium oxide samples
⑶Process XRD data and analyze scanning electron microscope images, and summarize relevant conclusions.
1.2 Experimental preparation
1.2.1 Experimental drugs
1.2.3 Determination of experimental parameters
Using nitrate and carboxylate system, magnesium nitrate hexahydrate is used as precursor. The mechanism of action of citric acid, the effect of different process conditions (water, anhydrous ethanol, and the amount of citric acid added) on the stability of sol-gel, and the effect of roasting temperature on the particle size and crystallinity of powder crystals were studied.
According to water: magnesium nitrate hexahydrate: citric acid: ethanol = 100:9:9:2.1 (molar ratio)
Based on 25ml water as the standard and the relevant data, we calculated:
2 Experimental process of nano-magnesium oxide
2.1 Experimental process
2.1.2 Specific experimental process
⑴ Mix 25ml water, 32g magnesium nitrate hexahydrate, 26.25g citric acid, and 1.725ml ethanol to make them uniform. Stir the solution at 50℃
for 30 minutes.
⑵ Set the temperature to 80℃ and stir for 2 hours.
⑶ After standing for at least 5 hours, the solution changes from sol to gel with increased viscosity and slightly yellow.
⑷ Put the gel that has been stationary for a long time into a roasting furnace and roast it at 500℃ for 3 hours. The gel becomes loose white honeycomb and breaks into white powder when touched.
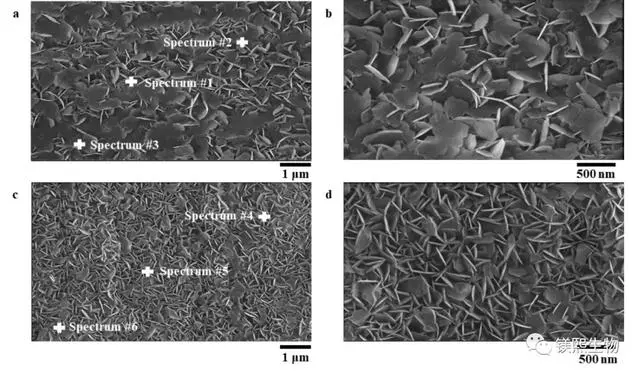
⑸ Electron microscope scanning analyzes its phase state, and XRD analyzes the particle size and related scientific parameters.
2.2 Problems in the experiment
In the early experiments, the magnesium oxide obtained by roasting contained some impurities. This problem could not be solved by extending the roasting time. The experiment found that due to the limited experimental conditions, the temperature of the roasting furnace was limited and the temperature control device was unstable. In the entire experiment, the colloid formation environment was limited and could only be controlled artificially.
2.3 Reactions in the experiment
Since Mg2+ and citric acid have strong complexing ability, magnesium citrate will be generated first. The precursor of magnesium citrate is relatively complex and contains polymers. At the same time, Mg2+ will hydrolyze as follows: Mg2++2OH-Mg(OH)2
3 Preparation method and experimental data of nano-magnesium oxide
3.1 Preparation of nano-magnesium oxide
Using magnesium nitrate hexahydrate as a precursor, nano-magnesium oxide powders with different particle sizes (10-100nm) and crystallinity were synthesized by citric acid sol-gel method. The mechanism of action of citric acid, the effect of different process conditions (water, anhydrous ethanol, amount of citric acid added) on the stability of sol-gel, and the effect of calcination temperature on the particle size and crystallinity of powder crystals were studied. The results show that when citric acid is not introduced, the stability of the gel is poor. The particle size of the magnesium oxide product is large and the agglomeration is serious. After the introduction of citric acid, a stable gel system can be formed when water: magnesium nitrate hexahydrate: citric acid: ethanol = 100: 9: 9: 2.1 (molar ratio). When the formed gel is heated, a redox reaction occurs, in which NO-3 provides an oxidizing atmosphere and COO-3 serves as a fuel. NO-3 and COO-3 in the gel structure undergo an “in-situ” oxidation-reduction reaction at a certain temperature, resulting in self-propagating combustion. Until all the dry gel is burned out, a loose powder is formed.
3.2 Characterization of nano-magnesium oxide and its precursors
Compared with the standard spectrum of magnesium oxide, the XRD spectrum of the powder sample shows that the peak position is completely consistent with the peak position of the standard spectrum (2θ=43.00°, 62.40°, 78.70°, 37.00°, 74.80°), indicating that the sample prepared in the experiment is MgO crystal, and no other impurity crystals are generated. The average particle size of the powder is calculated to be 41nm by the Scherrer formula.
4 Experimental results and analysis
4.1 Experimental results
The nano-powder prepared by the sol-gel method has a narrow particle size distribution, good dispersibility, high purity, easy reaction control, few side reactions, simple process operation, and low calcination temperature, which can greatly reduce the cost of raw materials.
The particle size obtained by the sol-gel method tends to be consistent. The sol-gel method can control the microstructure of the material in the early stage of material preparation. The sol-gel method has broad application prospects in nano-oxides.
4.2 Result Analysis
4.2.1 Foaming Phenomenon
When the precursor mixture is stirred at 80℃, a large number of bubbles will be generated, and a foamy anhydrous gel will be formed as the standing time increases. The foaming phenomenon is more obvious when calcined at 500℃.
Based on a large number of experiments, we found that the foaming temperature is related to the viscosity of the precursor mixture sol. Generally speaking, foaming can occur when the concentrations of nitrate and citric acid are relatively small. Through multiple groups of experiments, we know that foaming is directly related to the concentration of citric acid, and has little to do with the concentration of nitrate. The annual of the colloid is mainly determined by citric acid. The higher the concentration of citric acid, the greater the viscosity, and the higher the foaming temperature.
Foaming is a basic characteristic of sol. The phenomenon is the expansion of the sol volume, and a porous foam-like anhydrous dry gel is obtained. The colloid will foam during the heating process.
4.2.2 How to prevent agglomeration
⑴ Citric acid, as a typical complexing agent, forms a complex with metal ions and then undergoes the sol-gel process, but the metal ions are evenly distributed in the gel.
⑵ Citric acid is a hydroxycarboxylate with a low molecular weight. Some carboxyl groups can replace the abundant hydroxyl groups on the surface of the oxide, and combine with metal ions to form a monomolecular adsorption layer, so that the surface of the particles is negatively charged and repel each other, playing a dispersing role.
⑶ The foaming process can effectively control the agglomeration of powders by preventing the formation of chemical bonds, preventing network collapse and reducing the self-propagating temperature.
5 Conclusion
⑴ The sol-gel method can obtain nano-magnesium oxide powders with uniform grain size, good dispersion and high crystallinity, and at the same time lays the foundation for the future development of nano-magnesium oxide film materials.
⑵ The intervention of citric acid makes the reaction system more stable and plays a good dispersing role.
⑶ Nano-magnesium oxide is prepared by precipitation method. The raw materials are cheap, the process is simple, and the equipment requirements are not high. It also has low energy consumption and is suitable for industrial production. The prepared nano-magnesium oxide is evenly dispersed.

