Abstracts: MgCO3-3H2O crystals were prepared by co-precipitation method using MgCl2-6H2O as the raw material and Na2CO3 as the precipitant to explore the effects of reactant concentration, reaction temperature, reaction time and aging time on the composition and morphology of the products, and to investigate the growth mechanism. The results showed that the rod-like MgCO3-3H2O crystals with an average diameter of about 5 μm could be obtained at a reactant concentration of 0.3 mol/L, a reaction temperature of 50 ℃, a reaction time of 15 min, and an aging time of 0.5 h. The growth of MgCO3-3H2O crystals was in accordance with the liquid-liquid-solid growth mechanism.
Keywords: magnesium carbonate trihydrate; coprecipitation; preparation; morphology
Magnesium carbonate trihydrate (MgCO3-3H2O) crystals are single crystals of magnesium carbonate, the crystallization of the atomic structure of the highly ordered arrangement of the crystal, the crystal internal defects, the strength of the value of the crystal is close to the ideal, excellent physicochemical properties, is often used as rubber, plastics and other polymers of the toughness of the reinforcement agent and ceramics and other refractory additives, with a very high industrial value. Because of the high purity and thermodynamics of the sub-stable phase and other characteristics, magnesium carbonate trihydrate crystals are often used as the production of basic magnesium carbonate, magnesium oxide, anhydrous magnesium carbonate and other stabilized phase of fine magnesium salts of intermediate products.
At present, the common preparation methods of magnesium carbonate trihydrate whiskers include coprecipitation, hydrothermal method and carbonation method. Botha et al. prepared MgCO3-3H2O by adding CO2 into Mg(OH)2 suspension and warming it up, and investigated the effects of reaction temperature, pH and hydrochloric acid on the morphology and structure of MgCO3-3H2O whiskers.Wang et al. used MgCl2-6H2O and (NH4)2CO3 as the reactants, and controlled the initial concentration of the reactants and reaction temperature to prepare MgCl2-6H2O with a length of 40 μm, and then prepared MgCl2-6H2O with a length of 40 μm. MgCO3-3H2O whiskers with a length of 40 μm were prepared, and the products were calcined at 800 ℃ to obtain high-purity MgO. Gao Yujuan et al. obtained rod-like, radial and granular MgCO3-3H2O crystals by varying the concentrations of ammonium bicarbonate and magnesium ions using MgCl2-6H2O and NH4HCO3 as reactants. Zhao Bin et al. used MgSO4-7H2O and Na2CO3 as raw materials, and synthesized rod-shaped MgCO3-3H2O crystals with good crystallinity when the temperature was 50 ℃, the concentration of MgSO4 was 1.2 mol/L, and the ratio of MgSO4 to Na2CO3 was 1∶1.2, and the reaction time was 40 min.
Shen Rui et al. prepared well-dispersed magnesium carbonate trihydrate crystals using pretreated boron sludge as raw material, sodium carbonate as precipitant and disodium hydrogen phosphate (Na2HPO4-12H2O) as additive. Wang Yulian et al. used magnesite as raw material, calcination-hydration-carbonation-pyrolysis method, and sodium dodecyl sulfate (SDS) as crystal control agent to prepare rod-shaped MgCO3-3H2O crystals with an average aspect ratio of 25. Ou Long et al. light burnt dolomite powder as raw material, after digestion, carbonization to prepare heavy magnesium water precursor solution, and to the Mg (HCO3)2 solution into the air, pyrolysis under the condition of 60 ℃ to obtain MgCO3-3H2O whiskers. Taking industrial magnesium-containing substances such as boron mud, salt lake brine and natural minerals (magnesite, dolomite, etc.) as raw materials, it has certain guiding significance for the industrialized production of magnesium carbonate trihydrate, but the process factors are still cumbersome.
Throughout the research results at home and abroad, the crystal morphology of the prepared magnesium carbonate trihydrate is mostly rod-like. The author’s group in the early magnesite as raw material, light burning to obtain high activity MgO, and high activity MgO by hydration to produce Mg(OH)2 suspension, with hydrochloric acid acid leaching Mg(OH)2 suspension and add precipitant, synthesized MgCO3-3H2O crystals. In this paper, on the basis of the previous work, MgCO3-3H2O crystals were prepared by co-precipitation method using the chemical reagents MgCl2-6H2O and Na2CO3 as raw materials, and the effects of reactant concentration, reaction temperature, reaction time, aging time, and the additive sodium citrate on the physical composition and microscopic morphology of the crystal products were examined, and the effect of MgCl2-6H2O-Na2CO3 on the physical composition and microscopic morphology of the crystal products was investigated, as well as the effect of MgCl2-6H2O-Na2CO3 on the physical composition and microscopic morphology of the crystal products were investigated. and investigated the growth mechanism of MgCl2-6H2O-Na2CO3 in MgCl2-6H2O-Na2CO3 system.
1 Experiment
1.1 Main reagent materials
Magnesium chloride (MgCl2-6H2O, analytically pure, Tianjin Damao Chemical Reagent Factory); sodium carbonate (Na2CO3, analytically pure, Tianjin Komeo Chemical Reagent Co., Ltd.); sodium citrate (Na3C6H5O7-2H2O, analytically pure, Shenyang Dongxing Reagent Factory).
1.2 Methods
1) Sample preparation
A certain volume of MgCl2-6H2O solution with a concentration of 0.05-0.30 mol/L was placed in a beaker, and the beaker was kept warm in a thermostatic water bath at 20-50 ℃. According to the material quantity ratio of 1:1, the same volume and concentration of Na2CO3 solution was taken at the same temperature, and then it was poured into the beaker with MgCl2-6H2O solution at one time with continuous stirring, and the stirring was stopped at the end of the reaction, and the reaction was aged for a certain period of time under the condition of room temperature, and then filtered, and then the obtained white precipitate was dried in a constant temperature drying oven at 50 ℃ for 4 h, and then a white powder was obtained.
2) Characterization of the sample
MIT500 metallurgical microscope from Chongqing Aote Optical Instrument Co., Ltd. was used to observe the microscopic morphology of the products. The sample was detected by an Ultima IV X-ray diffractometer produced by Rigaku, Japan, with a radiation source of Cu-Cu target Kα, λ=0.154 1 nm, a solid detector, a tube voltage of 40 kV, a tube current of 40 mA, a scanning rate of 12 (°)-min-1, and a scanning range of 2θ=5 ° to 90 °. The sample was characterized by a Hitachi S-340000 X-ray diffractometer, which was used for the observation of the microstructure of the product. Hitachi S-3400N scanning electron microscope was used to observe the morphology of the products after the gold spraying treatment.
2 Results and discussion
2.1 Effect of reactant concentration
The product morphology was obtained at a fixed reaction temperature of 50 ℃, a stirring rate of 300 r/min, a reaction time of 15 min, an aging time of 0.5 h, and reactant concentrations of 0.05, 0.10, 0.20, and 0.30 mol/L in Fig. 1. As can be seen from the figure, the product was mainly irregular agglomerates with a small amount of rod-like crystals. At 0.05 mol/L, the product was mainly irregular agglomerates with a small number of rod-shaped crystals, as shown in Fig. 1 (a); at 0.10 mol/L, needle-shaped crystals started to precipitate, but the crystal length was short and the yield was low, as shown in Fig. 1 (b); at 0.20 mol/L, the surface of the rod-shaped crystals was rough; some small nuclei of the particles in the reaction solution clustered together in order to reduce the surface energy of the particles and a small number of agglomerates were produced, as shown in Fig. 1 (c); When the concentration of the reactants was increased to 0.30 mol/L, all the products were smooth and homogeneous rod-shaped crystals, as shown in Fig. 1 (d).
It can be seen that the growth and morphology of the product are strongly influenced by the concentration of the reactants. At 0.10 mol/L, the concentration of ions in the solution is low, the degree of supersaturation is small, the nucleation is incomplete, and the resulting whiskers are few in number and short in length, so the appropriate concentration of reactants is 0.30 mol/L. The product was found to be smooth and homogeneous.
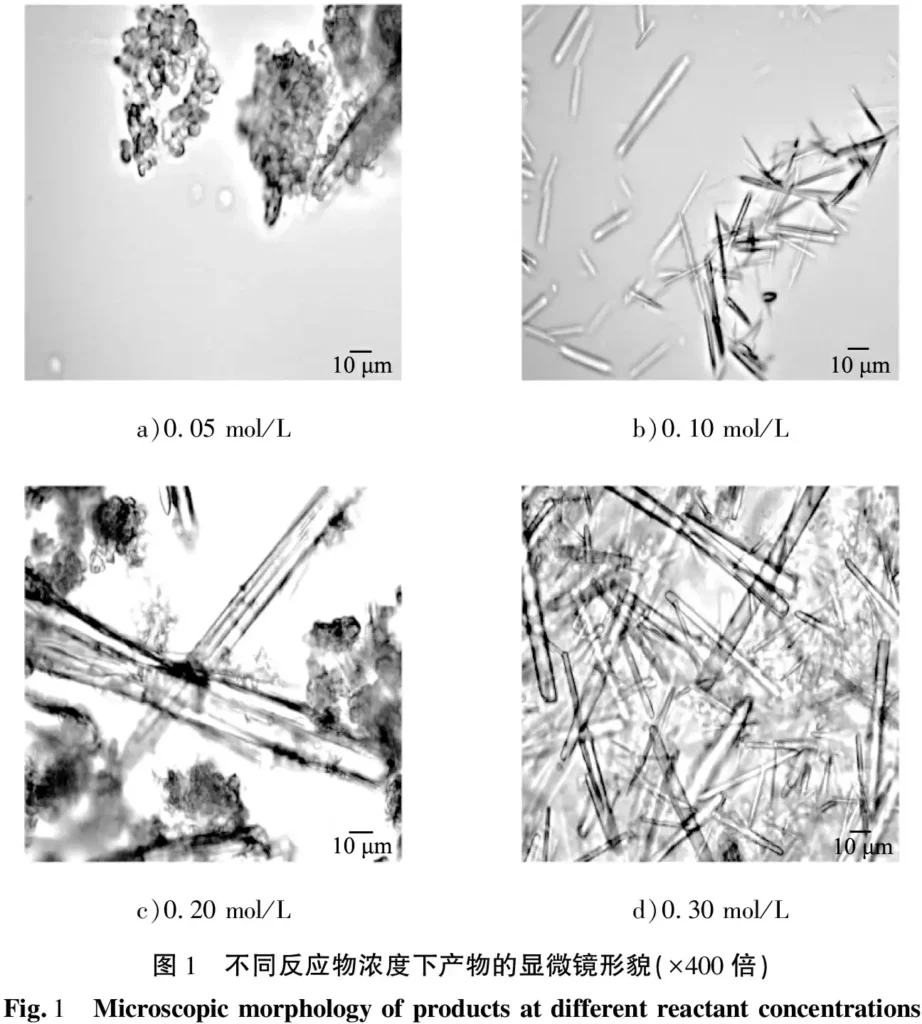
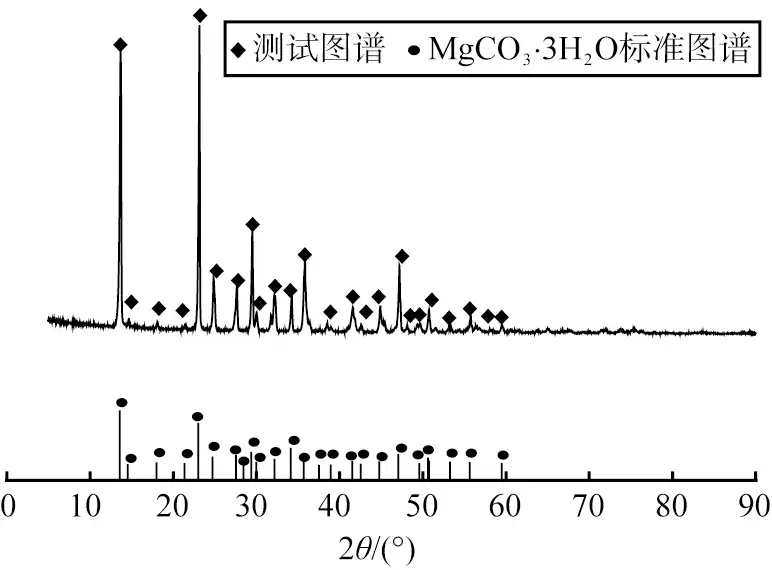
Figure 2 shows the XRD image of the product obtained at a concentration of 0.30 mol/L. The diffraction peaks of the product matched the characteristic peaks of MgCO3-3H2O (PDF#70-1433). The sharp shape of the diffraction peaks, flat base and absence of other impurity peaks indicate that the product is of high purity and has fewer impurity phases, therefore, the concentration of 0.30 mol/L is suitable for the reaction.
2.2 Effect of reaction temperature
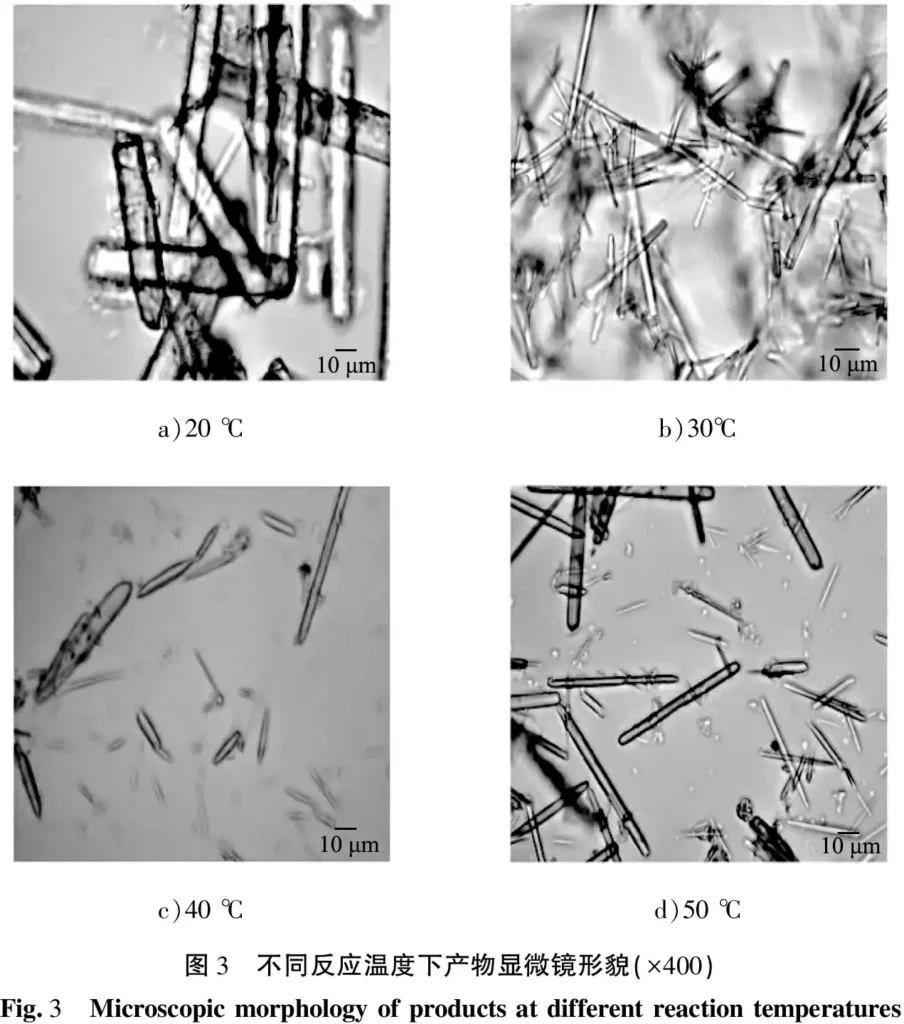
The morphology of the products obtained at a fixed reactant concentration of 0.30 mol/L, a stirring rate of 300 r/min, a reaction time of 15 min, an aging time of 0.5 h, and reaction temperatures of 20, 30, 40, 45, and 50 ℃ are shown in Fig. 3. As can be seen in the figure, when the reaction temperature was 20 ℃, the products were coarse rod-shaped crystals as shown in Fig. 3 a) under the 400× objective microscope but the quantity was small. When the reaction temperature was increased to 30 ℃, the products were fine rod-shaped crystals, see Figure 3 b); when the reaction temperature was 40 ℃, the length of the crystals obtained was uneven, and the yield was small, see Figure 3 c); and when the temperature was continued to be increased to 50 ℃, the rod-shaped crystals of longer length were obtained, see Figure 3 d). 20 ℃, the reaction temperature of the reaction system was low, the reaction rate was slow, and there was little nucleation, which resulted in the final product being coarse.
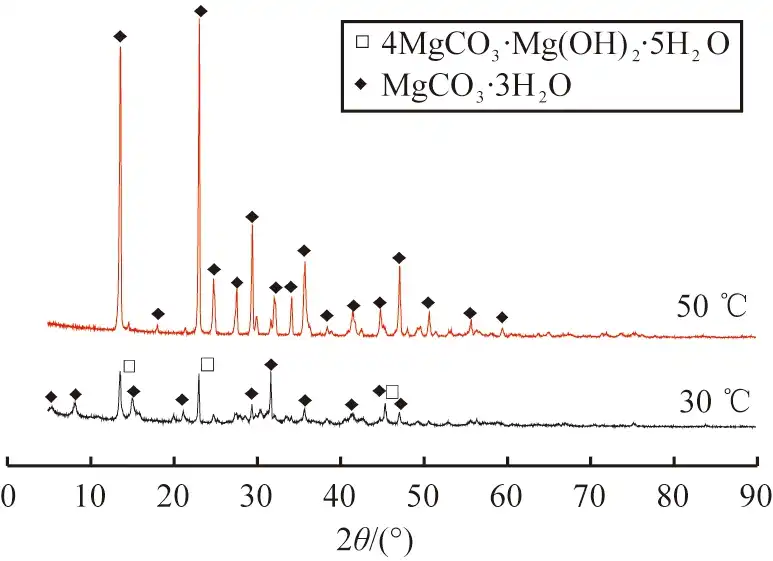
The XRD results of the products obtained at 30 ℃ and 50 ℃ are shown in Figure 4. It was found that the characteristic peaks of 4MgCO3-Mg(OH)2-5H2O and MgCO3-3H2O existed in the diffraction peaks of the products at 30 ℃, indicating that the products obtained at this temperature were a mixture of the two. At 50 ℃, the diffraction peaks of the products matched with the characteristic peaks of MgCO3-3H2O, with high peaks and no other peaks, indicating that high purity and good crystallization of rod-shaped MgCO3-3H2O crystals could be obtained at this temperature, so 50 ℃ was chosen as the optimum reaction temperature.
2.3 Effect of reaction time
When the reactant concentration is fixed at 0.30 mol/L, the stirring rate is 300 r/min, the reaction temperature is 50 ℃, the aging time is 0.5 h, and the reaction time is 5, 10, 15, 20, 25 and 40 min, respectively, the XRD patterns of the obtained products are shown in Figure 5. As can be seen from the figure, when the reaction time is between 5 and 15 min, the obtained products are all MgCO3·3H2O. When the reaction time is 15 min, the base of the product XRD image is smooth, the peak is narrow and sharp, and the diffraction peak of the product is consistent with the characteristic peak of MgCO3·3H2O, without other impurity peaks, indicating that the product obtained under this reaction condition has high crystallinity and few impurities; when the reaction time is 20 min, there are two diffraction peaks in the XRD spectrum at the same time, and the obtained product is a mixture of MgCO3·3H2O and 4MgCO3·Mg(OH)2·4H2O. At 25 and 40 min, the characteristic peaks of the product were consistent with the standard peaks of 4MgCO3·Mg(OH)2·4H2O (PDF#70-0361), and the product was basic magnesium carbonate.
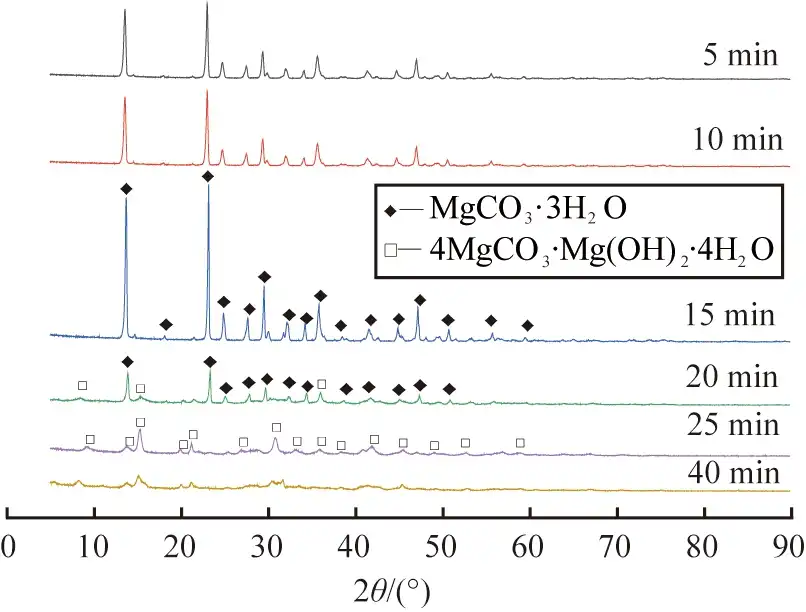
Figure 6 shows the SEM images of the products obtained at different reaction times. As can be seen from Fig. 6 a), the products were mainly short and thick columnar crystals with uneven crystal distribution at the reaction time of 5 min; when the reaction time was prolonged to 10 min~15 min, the products were rod-shaped crystals with smooth surfaces, see Fig. 6 b) and c), in which the average length of the products obtained at 15 min was about 60 μm; when the reaction time was continued to be increased to 20 min, flocculent materials were started to be produced on the surface of the rod crystals, which might be caused by the partial dissolution of rod crystals, see Fig. 6 d); at the reaction time of 25~40 min, the rod crystal surfaces began to generate When the reaction time continued to increase to 20 min, the surface of the rod-shaped crystals began to produce flocculent material, which may be due to the partial dissolution of the rod-shaped crystals, see Fig. 6 d); when the reaction time ranged from 25 to 40 min, the rod-shaped crystals continued to dissolve and were completely converted into flocculent crystals, see Fig. 6 e) and f).
In summary, the reaction time has an important effect on the morphology and physical phase composition of the product, with the increase of the reaction time, the product is gradually converted from magnesium carbonate trihydrate to basic magnesium carbonate; combined with the results of XRD and SEM analysis, the appropriate reaction time for the preparation of MgCO3-3H2O whiskers was determined to be 15 min.
2.4 Effect of aging time
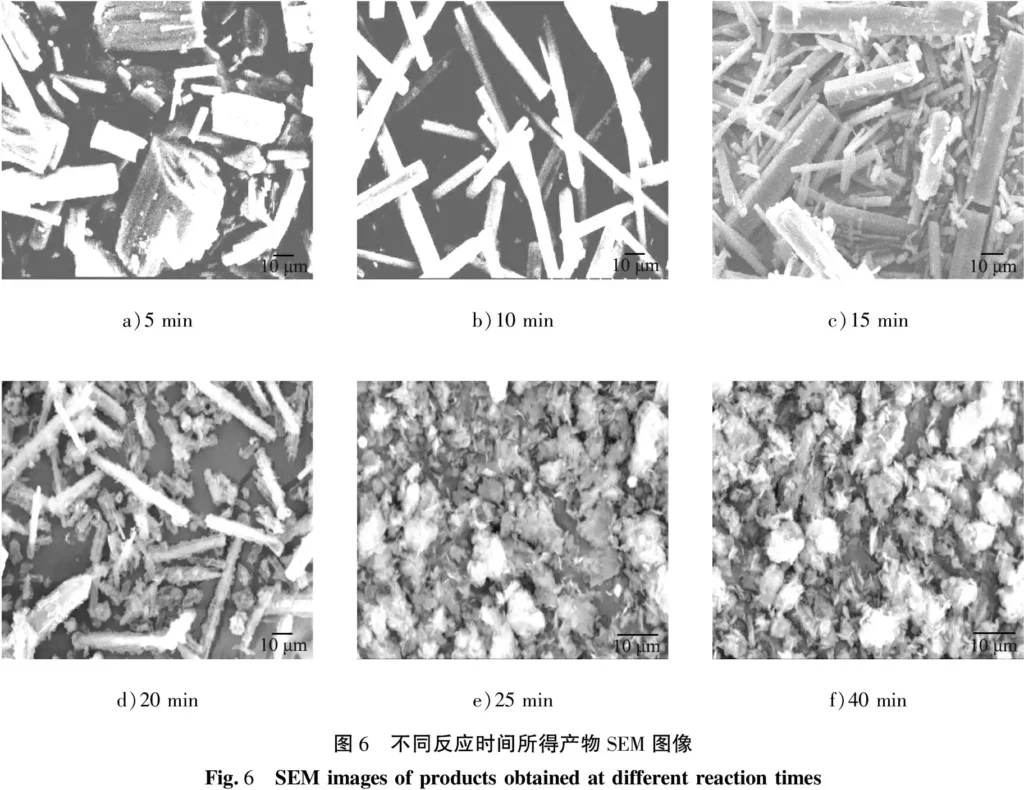
When the concentration of the fixed reactants was 0.30 mol/L, the stirring rate was 300 r/min, the reaction temperature was 50 ℃, the reaction time was 15 min, and the aging time was 0, 0.5, 1, 3 and 6 h, the morphology of the obtained products was shown in Fig. 7. When the aging time was 0 h, the growth time of amorphous particles in the solution was insufficient to form more crystals, resulting in a smaller number of products. As can be seen from the figure, when the aging time is 0.5 h, the products are rod-shaped crystals with smooth surface and uniform length; with the extension of aging time to 1 h, there is no obvious change in the morphology of the products, and the number of crystals does not increase significantly; when the aging time continues to extend to 3-6 h, there are both rod-shaped crystals and flocculent material in the products. According to the results of the group’s previous research work, it can be seen that the flocculent material is basic magnesium carbonate. This is because magnesium carbonate trihydrate is substable hydrated magnesium carbonate, which will be converted to thermodynamically more stable alkaline magnesium carbonate under certain conditions. Considering the product yield and other factors, 0.5 h was determined as the appropriate aging time.
2.5 Effect of adding sodium citrate
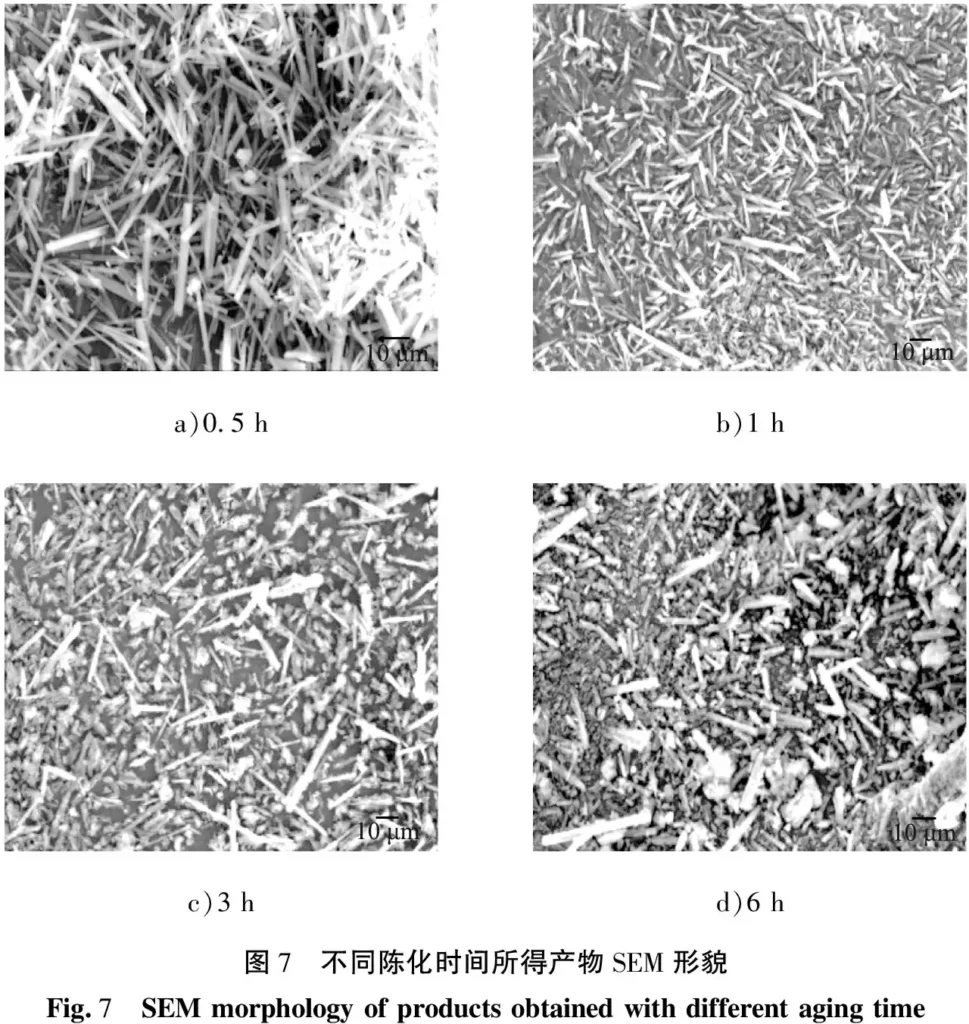
Fixed temperature of 50 ℃, stirring rate of 300 r/min, aging time of 0.5 h, the addition of sodium citrate 2% (according to the volume fraction of the reaction solution), the reaction time of 45 min, to explore the effect of sodium citrate on the composition and morphology of the product, the results are shown in Figures 8 and 9. The results are shown in Figs. 8 and 9. As can be seen from the figures, the morphology of the product obtained after the addition of sodium citrate was radial, and the XRD spectrum of the product had a gentle base, a narrow half-broad peak and a high intensity, and the product was still a crystal of MgCO3-3H2O, which indicated that the addition of sodium citrate did not change the composition of the product, but had a significant effect on the morphology of the crystal, which may be due to the selective adsorption of sodium citrate, which promotes the growth of the crystal anisotropy, leading to the growth of the crystal of Mg(III) carbonate to a few specific crystals. The reason may be that the selective adsorption of sodium citrate promotes the anisotropic growth of crystals, resulting in the growth of magnesium carbonate trihydrate crystals to a few specific crystal faces.
2.6 Whisker growth mechanism
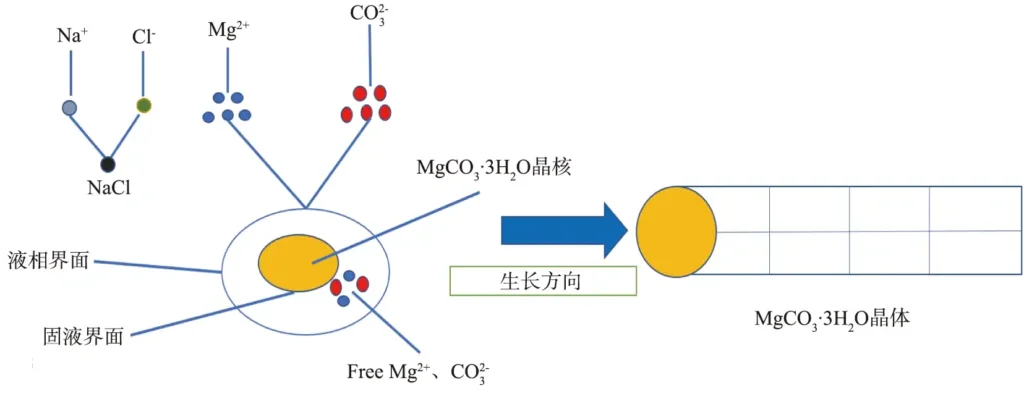
A certain concentration of Na2CO3 solution added to the same concentration of MgCl2-6H2O solution, the reaction solution supersaturation increases rapidly, a large number of MgCO3-3H2O crystal nuclei precipitation, mutual attraction and aggregation, and along the solid-liquid interface can be the direction of the lowest one-dimensional growth; at the same time, the loss of electrons outside the nucleus of the Na+ and the get electrons of the Cl– in the electrostatic attraction of the combination. During the growth of rod-shaped MgCO3-3H2O crystals, the free Mg2+ and width=31,height=20,dpi=110 in the reaction system continuously gather to the solid-liquid interface to provide growth material, and it is inferred that the growth of MgCO3-3H2O whiskers is in accordance with the liquid-liquid-solid growth mechanism, which is schematically shown in Figure 10.
3 Conclusion
1) When the concentration of MgCl2-6H2O was 0.30 mol/L, the concentration of Na2CO3 was 0.30 mol/L, the reaction temperature was 50 ℃, the reaction time was 15 min, the stirring rate was 300 r/min, and the aging time was 0.5 h, the rod-shaped MgCO3-3H2O crystals with an average diameter of about 5 μm were obtained.
2)The reaction time has a great influence on the crystal preparation process. When the reaction time is prolonged (>15 min), the rod-shaped magnesium carbonate trihydrate crystals are transformed into flocculent basic magnesium carbonate.
3)When the addition amount of sodium citrate in the MgCl2-6H2O-Na2CO3 system was 2%, well-crystallized radial MgCO3-3H2O crystals could be obtained.
4) In the MgCl2-6H2O-Na2CO3 system, the MgCO3-3H2O crystals grew one-dimensionally along the solid-liquid interfacial energy in the direction of the lowest, which is presumed to be in accordance with the liquid-liquid-solid growth mechanism.
