Hebei Messi Biology Co., Ltd. uses three different chemical processes to prepare hexagonal flake magnesium hydroxide with good dispersion performance and flame retardant effect.
The first process is a two-step preparation method of magnesium oxide hydration and hydrothermal. The hydrothermal medium uses weak alkaline substances. The raw magnesium oxide is first hydrated to prepare a crude magnesium hydroxide product; then a certain concentration of weak alkaline medium sodium acetate and sodium citrate are added to the crude product for hydrothermal treatment. The high temperature and high pressure during hydrothermal treatment can increase the solubility of magnesium hydroxide. After a long dissolution-crystallization process, the magnesium hydroxide particles precipitated again have regular morphology and are hexagonal flakes.
In addition, Hebei Messi Biology Co., Ltd. found that different types of surfactants and different dosages have different degrees of influence on the morphology and particle size. Different surfactants have different mechanisms of action. By comparison, it is found that PVP molecules have the best effect because they have dual effects, both steric hindrance and coordination with magnesium ions.
The second process also uses a two-step method of magnesium oxide hydration and hydrothermal to prepare hexagonal flake magnesium hydroxide. First, the crude magnesium hydroxide product is prepared by hydration; then a certain concentration of organic medium is added for hydrothermal reaction. The addition of organic solvents has a significant effect on the pressure of the system, and the pressure of the system directly determines the rate of chemical reactions. In this system, various organic solvents adjust the reaction rate by adjusting the system pressure, and because the organic solvents are relatively viscous, they reduce the mass transfer rate of ions in the system.

Hebei Messi Biology Co., Ltd. has found that alcohols, amines, and alcoholamines have the ability to complex Mg2, which can increase the solubility of crude products in hydrothermal reactions. The higher the solubility, the better the crystallinity of the crystals generated by recrystallization. A certain concentration of OH- can promote the formation of unit elements, so the alkalinity and polarity of the mixed solution will affect the crystallinity of the crystals to varying degrees, making the morphology of the crystals tend to be regular.
The third process calcines the finished product prepared by the first process to explore the effects of different calcination times and calcination temperatures on the particle size and morphology of the product magnesium oxide. The calcined magnesium oxide is hydrated again to explore whether different hydration times and temperatures can directly convert magnesium oxide into hexagonal magnesium hydroxide.
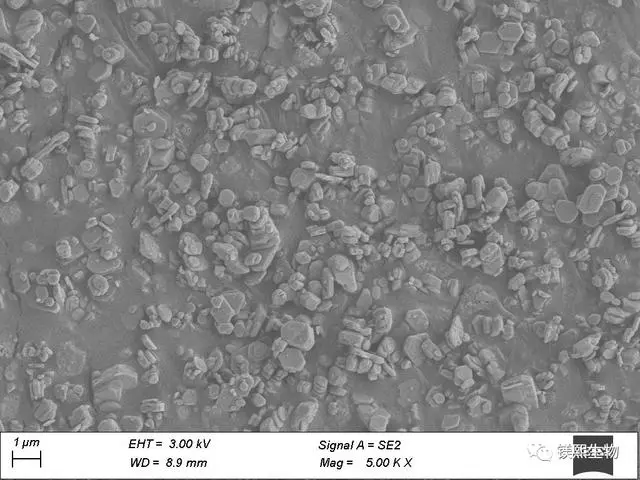
Hebei Messi Biology Co., Ltd. has found that as the calcination temperature increases, the grain size increases, but the rate of increase changes. When the temperature rises from 650°C to 750°C, the grain size of magnesium oxide changes from a slow increase to a sudden increase. If the calcination time of magnesium hydroxide is short, the decomposition is not complete. If the time is too long, it will form a dead burn, lose the activity of magnesium oxide, and cannot be hydrated again to form hexagonal magnesium hydroxide. Finally, it was found that the magnesium oxide generated at a calcination temperature of 550°C and a time of 6h is most conducive to the one-step conversion into hexagonal magnesium hydroxide.
