Hebei Messi Biology Co., Ltd. stated that hydrothermal synthesis is a process in which aqueous solutions undergo chemical reactions under certain pressure and temperatures exceeding the boiling point. Water has two main functions here: (1) to partially dissolve reagents, and (2) to transmit pressure. Many different aqueous solutions can simultaneously serve as solvents and provide reaction environments. The main advantage of hydrothermal synthetic materials is that in addition to good control over the size and morphology of the crystal grains, it can also prevent agglomeration, resulting in well-dispersed materials.
The magnesium oxide precursor is usually obtained hydrothermally in a stainless steel autoclave, lined with a thin layer of polytetrafluoroethylene for corrosion protection. Put the magnesium-containing raw materials in the reactor, then add a solvent of about 70% of the total volume of the reactor, seal the autoclave tightly, and finally heat the reaction at high temperature for a period of time. After the reaction is completed and cooled, the reacted product is passed through a series of The operation yields magnesium oxide. The crystal grain size of the magnesium oxide precursor synthesized by the hydrothermal method is 10nm-50nm, and the specific surface area is greater than 100m2/g.
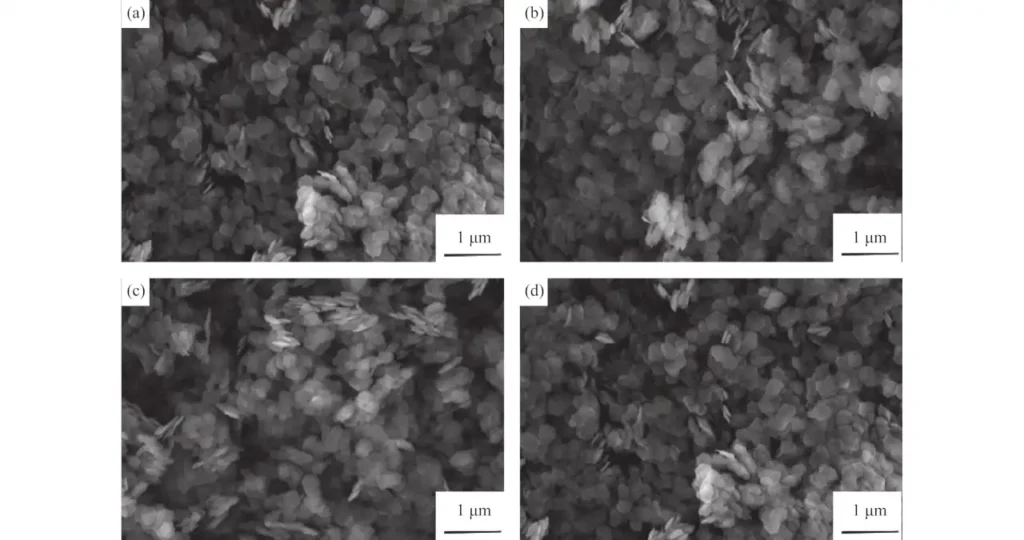
This method not only effectively controls the size and shape of the obtained product, but also analyzes the effects of magnesium salt, solvent and process parameters on the performance of the magnesium oxide precursor. Study the relationship between the morphology of the product and pH and temperature. Experiments have shown that the morphology of magnesium oxide particles can be controlled by adjusting the pH value during the hydrothermal process. For example, by changing the pH, flowers, needles, and other shapes can be synthesized. Platelet-shaped and other irregular spherical forms of magnesium oxide.
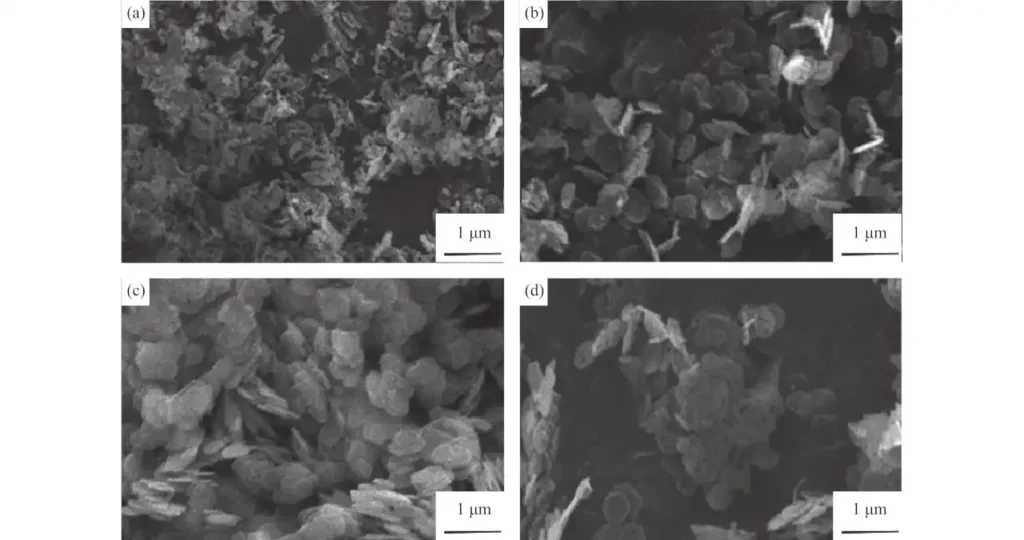
A hydrothermal method for synthesizing various metal hydroxides using commercial metal oxides as raw materials is described. This solid-solution-solid conversion efficiency is as high as 100%. The magnesium hydroxide and magnesium oxide prepared by this process have a large specific surface area and can be used as a catalyst or catalyst carrier.
Using natural brucite and magnesium lactate as raw materials, porous magnesium oxide nanosheets are directly synthesized through a hydrothermal method. The pore diameter of the synthesized magnesium oxide is approximately 8.3nm, which is ultimately used as a catalyst. Nanoscale hexagonal magnesium hydroxide was prepared by the ammonia-hydrothermal method, that is, during the ammonia precipitation and hydrothermal processes, the crystallinity and dispersion of the magnesium hydroxide particles were improved by adding citric acid and monoethanolamine, and the results were analyzed by scanning electron microscopy and X-ray diffraction. and thermogravimetric and other characterization methods to analyze the morphology, crystal structure and thermal stability of the sample. The results show that well-dispersed hexagonal magnesium hydroxide nanosheets with an average diameter of 246 nm can be prepared through this newly developed process.
Hebei Messi Biology Co., Ltd. stated that compared with other methods, this method can improve the solubility of the product by accelerating the process of diffusion, adsorption and ionization, thereby speeding up the chemical reaction and improving the crystallinity of the material, but its drawback is High temperature conditions are required, which is not conducive to industrial production.

