A convenient method for the synthesis of rosette-shaped porous alkaline magnesium carbonate (4MgCO3-Mg(OH)2-4H2O) microspheres, which is divided into the synthesis of a precursor of magnesium carbonate trihydrate (MgCO3-3H2O) and its pyrolytic preparation in water, is presented. The precursor was synthesized by stirring-induced crystallization-assisted aging method, and the homogeneous microrods with a length of about 115 μm and an aspect ratio of about 10.4 were obtained. Pyrolysis of the microrods in water at 353.2 K gave rise to the rosaceous porous magnesium carbonate microspheres consisting of curved nano-sheets with the structure of house of cards, with the diameters of the microspheres being in the range of 30-60 μm, and an average of about 40 μm, with a good performance of the porous basic magnesium carbonate microspheres. The diameter of the microspheres was 30~60 μm, with an average of about 40 μm, and they had good dispersion. The morphology transformation and phase transfer process during pyrolysis were investigated, and the structure and morphology of the samples were characterized by XRD, FTIR and SEM.
The rose-like porous basic magnesium carbonate (4MgCO3-Mg(OH)2-4H2O) microspheres were synthesized as follows:
Precursor synthesis :
Stirring-induced crystallization-assisted aging was used to synthesize the precursors. The specific steps include mixing magnesium chloride and sodium carbonate at a certain molar ratio (e.g., 12~5:1) and stirring at a certain temperature (0~80°C) for 0.05~6 hours, supplemented by a certain aging time (0.1~48 hours). After stirring, the precipitate is obtained, and the precipitate is filtered or washed by centrifugation and then prepared for use, or it can go directly to the next step without drying.
Pyrolysis Preparation :
The prepared precursor (magnesium orthocarbonate) is put into hot water at 60~100°C, stirred and dispersed, and then left to hold or held at low stirring rate for 0.25~12 hours. Subsequently, the product was discharged by filtration or centrifugation and dried at 60~110°C to obtain porous rosette-like basic magnesium carbonate microspheres.
Morphology and structure:
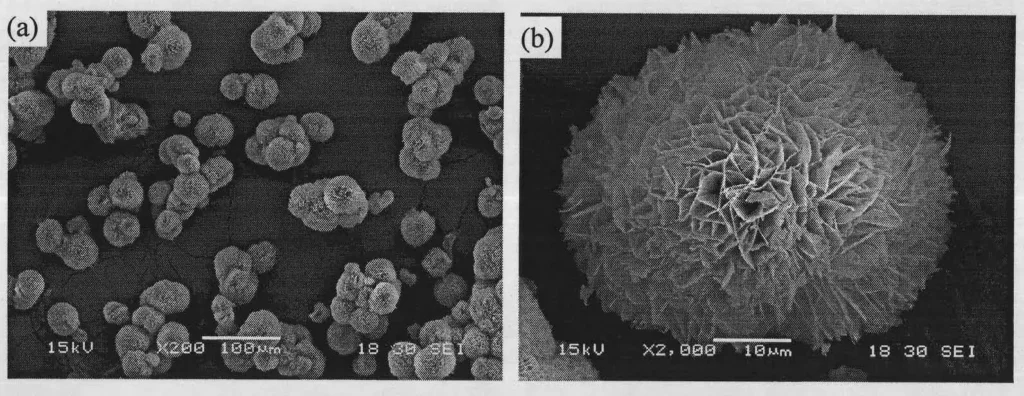
The synthesized precursors were homogeneous microrods, about 115 μm long, with an aspect ratio of about 10.44.
After pyrolysis in water at 353.2 K, rosette-like porous magnesium carbonate microspheres with a “card-box” structure consisting of curved nanosheets were obtained.4 The diameters of the microspheres were in the range of 30-60 μm, with an average of about 40 μm, and they were well dispersed.
Characterization method :
Characterization methods such as XRD (X-ray diffraction), SEM (scanning electron microscope) and TR (transmission electron microscope) were used to analyze the structure and morphology of the samples in detail and to confirm the morphology transformation and phase transfer process during pyrolysis.
Through the above steps and conditions, rosette-like porous alkaline magnesium carbonate microspheres with excellent dispersibility can be successfully synthesized, which have promising applications in various fields.
The results showed that MgCO3-3H2O dissolved due to its instability at higher temperatures, formed local supersaturation, generated amorphous particles, and nucleated and crystallized into 4MgCO3-Mg(OH)2-4H2O nanosheets on the microrods. The nanosheets grow outward from the attachment site of the rod to form rosette-shaped microspheres, and the growth of the microspheres is accompanied by the dissolution of the rod, and the particles grown on different parts of the rod will leave different traces in the microstructure. It is analyzed that the pyrolytic transformation process is (MgCO3-3H2O) dissolution – amorphous material generation – (4MgCO3-Mg(OH)2-4H2O) crystallization process.

