Messi Biology states that in MLCC manufacturing, magnesium hydroxide (Mg(OH)₂) as a magnesium-based additive has similar effects to magnesium carbonate (MgCO₃) (ultimately transforming into MgO to exert its function). However, it differs significantly in terms of process adaptability, decomposition behavior, dispersibility, and its impact on microstructure. Magnesium hydroxide has the potential to replace magnesium carbonate in MLCCs, especially suitable for advanced ultra-thin layer and low-temperature sintering designs. However, its process window is narrower, requiring strict resolution of dispersibility and moisture release issues.
Core Mechanism of Action
Magnesium hydroxide decomposes into magnesium oxide (MgO) during sintering, the same final product as magnesium carbonate:
Chemical Formula: Mg(OH)₂ → MgO + H₂O (Decomposition temperature: ~350℃)
MgO functions by:
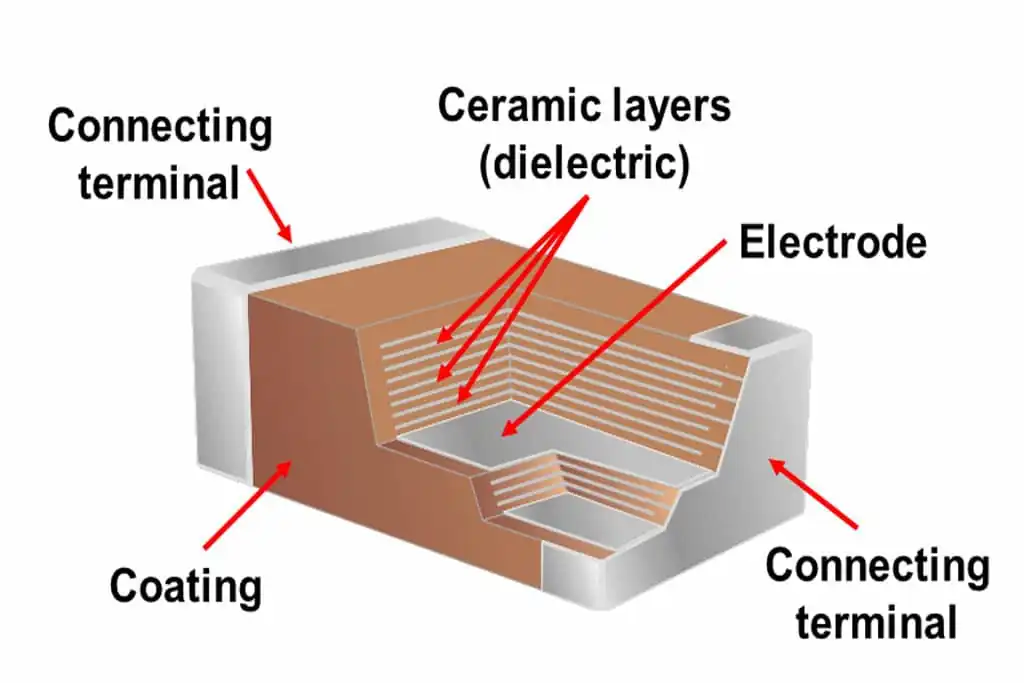
- Inhibiting Grain Growth: Mg²⁺ segregates at the grain boundaries of barium titanate (BaTiO₃), hindering grain boundary migration and refining grain size.
- Improving Dielectric Properties: Enhancing insulation resistance, reducing loss (tanδ), and stabilizing temperature characteristics.
- Adjusting Sintering: Suppressing excessive shrinkage and reducing deformation.
Unique Advantages of Magnesium Hydroxide
- Lower Decomposition Temperature: Magnesium hydroxide’s decomposition temperature (~350℃) is significantly lower than that of magnesium carbonate (~450℃).
- Advantage: Earlier generation of active MgO in the early stage of sintering, potentially dispersing more evenly in the grain boundaries, especially suitable for low-temperature sintering formulations (such as those matched with Cu or Ni electrodes).
- No CO₂ Release: The decomposition product is H₂O, avoiding the risk of CO₂ gas forming pores in the green body (magnesium carbonate releases CO₂, which may require slower heating rate control).
- Advantage: Reduces internal defects in the sintered body, improving density (needs to be matched with moisture removal processes).
- Potential Cost and Environmental Friendliness: The synthesis process of high-purity magnesium hydroxide may be simpler, with lower raw material costs. No carbon emissions, in line with green manufacturing trends.
Applicable Scenarios and Recommendations
- Recommended Scenarios for Trial:
- Ultra-thin dielectric layer MLCCs (<0.5μm): Clear need for low-temperature sintering, the early MgO generation of magnesium hydroxide may improve grain boundary uniformity.
- Low-Temperature Co-fired Ceramics (LTCC): Matching low-melting-point glass phases to avoid high-temperature decomposition interference.
- Pursuit of low cost/environmental protection: If the supply chain can stably provide high-purity products.
- Scenarios Requiring Careful Evaluation:
- Large-size/High-layer-count MLCCs: Difficult to remove moisture, high risk of cracking.
- Base Metal Electrode (BME) Systems: Strict prevention of H₂O oxidizing the electrodes.
- Existing Mature Magnesium Carbonate Process: High switching costs, requiring comprehensive verification of reliability.
- Key Success Factors:
- Powder Treatment: Nano-scale dispersion, surface hydrophobic modification.
- Process Adaptation: Optimize the binder burnout curve (slow heating + holding at 300-500℃).
- Purity Assurance: Anion impurities (Cl⁻, SO₄²⁻) must be <5ppm.
