Although hydromagnesite was discovered a long time ago, there has been no official report on it in China. At the same time, it is known that in Czechoslovakia, Italy, the United States, France and the Soviet Union, the mineral is produced in magnesian ultramafic rocks (alteration products of serpentine) and magnesite, for low-temperature hydrothermal and secondary products. It is accompanied by hydromagnesite, fibrous magnesite and serpentine.
We see hydromagnesite produced in the boromagnesite serpentine formed by the alteration of dolomitic magnesite marble. The enriched area is only a few meters away from the surface, which is caused by epigenetic effects. In this area, the fault development, magnesite serpentine is wrong into a breccia, and there are holes generated. Along the holes were successively filled with chrysoberyl and chlorite, and magnesite occurred in the unfilled residual holes. The formation environment of magnesite is free space, it mostly grows vertically or obliquely to the wall of the hole, and sometimes covered with a layer of calcite film, and the outermost is the residual holes. It is difficult to see it in the same alteration body of the wrong development of the section. Although magnesite is not a rare mineral, its distribution is very limited, and it is not a large-scale accumulation. Therefore, it is of some significance to discuss its mineral properties and formation conditions.
As a common water-containing magnesium-rich carbonate mineral, magnesite [Mg5(CO3)4(OH)2-4H2O] can be used as a high-quality mineral-type flame-retardant filler in the field of polymer flame retardancy. Studies have shown that hydromagnesite is mainly found in carbonate-type salt lakes and Quaternary lacustrine strata, and has also been found in the lacustrine deposits of the Jezero Crater of Mars, and the Mg/Ca value of the lake water is regarded as a key condition for hydromagnesite mineralization. The conventional view is that the formation of lake-phase hydromagnesite is mainly associated with the weathering of ultramafic rocks to provide Mg-rich source recharge, but the Mg/Ca values of surface river water and groundwater associated with the weathering of ultramafic rocks seldom reach a threshold sufficient to allow direct precipitation of hydromagnesite from salt lakes (Fig. 1a). Therefore, identifying the geochemical cycling processes of Mg in the salt lake system is a key component in deciphering the mineralization process of hydromagnesite.
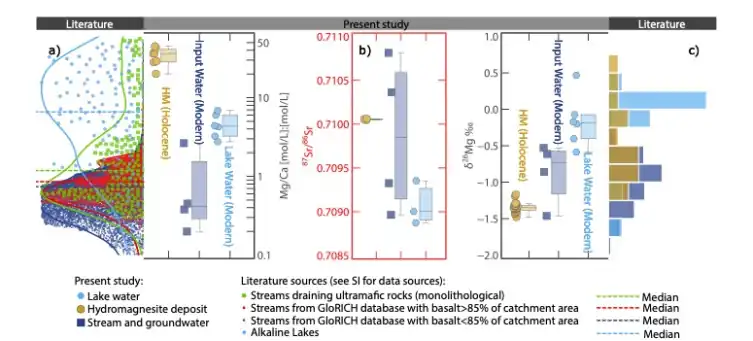
To address the above key scientific issues, based on the Field Scientific Observatory for Salt Lakes on the Tibetan Plateau of the Ministry of Natural Resources, Associate Researcher Lin Yongjie of the Institute of Mineral Resources of the Chinese Geological Survey (hereafter referred to as the “Institute”) and his co-workers systematically carried out Mg isotope studies of salt lake water, river water, groundwater and hydromagnesite in the example of the Dujiali Salt Lake of Tibet (Fig. 1c). Mg isotope study (Fig. 1c). In the present work, a semi-quantitative hydrochemical model of Mg/Ca and δ26Mg changes in Dujiali Salt Lake under evaporation conditions was developed by using Mg isotope analyses and the water chemistry simulation software Phreeqc (Fig. 2), and the geochemical cycling process of Mg in the salt lake system was investigated.
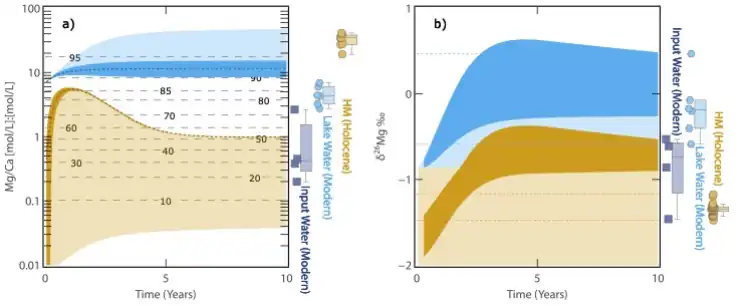
The results show that under strong evaporation conditions, under the evolutionary process of Lake Dujaili, the first saturated precipitation and precipitation out of the Chinese rock led to the increase of Mg/Ca and alkalinity of the lake water, which in turn drove the saturation and precipitation out of the hydromagnesite, and the Mg isotope provided a good parameter qualification for the model. The model results are further corroborated by the fact that in the vast majority of lake-phase hydromagnesite deposits globally, hydromagnesite is usually coeval or alternately deposited with aragonite. In summary, the present work proposes that although surface river water or groundwater associated with weathering of Mg-rich rocks provides an important source of Mg to salt lakes, precipitation of aragonite under evaporative conditions may have been an important metallogenic process driving the deposition of hydromagnesite in salt lakes.
