Composite metal hydroxides (LDHs), commonly known as hydrotalcite, are a typical two-dimensional layered anionic clay. Due to its unique layered structure, which has both the exchangeability of interlayer anions and the isomorphous substitution of cations in the layer, different types of LDHs are often synthesized by changing their ion species to give them different properties.
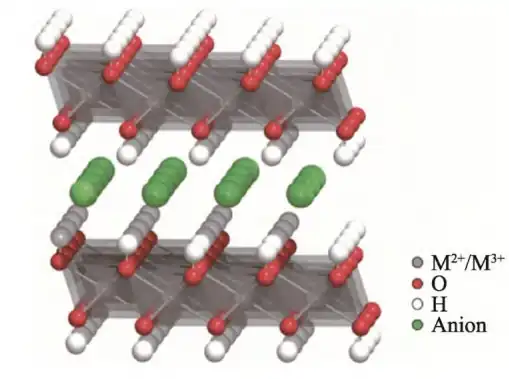
There are two types of hydrotalcite: natural and synthetic. Naturally occurring hydrotalcite is rare and difficult to obtain, but synthetic hydrotalcite has abundant sources of raw materials and simple process steps. After calcining, hydrotalcite compounds have good adsorption properties, anion exchange properties and catalytic activity. In addition, the structure of hydrotalcite compounds is controllable and adjustable, making them easy to utilize and process. They have been widely used in fields such as catalysis, adsorption, medicine, and flame retardancy.
1 Properties of hydrotalcite
1.1 Acid-base
Common MgAl-LDH and hydrotalcites are weakly alkaline compounds, and the alkalinity is enhanced by the formation of complex metal oxides (LDO) after high-temperature roasting.
1.2 Exchangeability
From the special layered structure of LDH, it can be seen that the interlayer anions can be exchanged with different kinds and quantities of anions.
1.3 Thermal stability
Hydrotalcite will decompose when heated, and the destruction of the ordered layered structure and the phenomenon of material transformation will occur when it is roasted at a certain temperature.
1.4 Memory effect
When the roasted MgAl-LDH is added into the solution containing certain anions, the destroyed layered structure of hydrotalcite can partially restore the ordered layered structure.
2 Hydrotalcite applications
2.1 Polymer materials
As a heat stabilizer, synthetic hydrotalcite has many advantages as a PVC heat stabilizer. Hydrotalcite itself is alkaline and can effectively absorb HCl (hydrogen chloride) released from PVC and prevent its further autocatalytic decomposition. Moreover, synthetic hydrotalcite is stable and compatible with Plastic has good compatibility. In addition, compared with toxic PVC heat stabilizers such as lead and cadmium, synthetic hydrotalcite has the advantages of being non-toxic and highly efficient.
As a flame retardant, synthetic hydrotalcite has the flame retardant advantages of magnesium hydroxide and aluminum hydroxide due to its special composition. The flame retardant function is mainly achieved through two methods: gas phase flame retardant and condensed phase flame retardancy.
As an acid absorbent in the production of spandex, synthetic hydrotalcite can maintain the elasticity (anti-oxidation) of clothing and extend its service life.
2.2 Water treatment
Due to the large specific surface area of hydrotalcite compounds and the exchangeability of anions between structural layers, they have good adsorption properties. The mechanism of adsorption of organic matter and cations is mainly surface adsorption, while the adsorption effect for anions is mainly due to interlayer anions. Exchangeability. At present, the biggest use is for adsorption of anionic pollutants in polluted wastewater.
Cation removal: Hydrotalcite can remove cations such as chromium ions, copper ions, and arsenic ions in water.
Anion removal, for high-salt wastewater containing a large amount of chloride ions, sulfate ions, fluoride ions, etc., by changing the reaction conditions, the concentration of pollutants can be reduced to the greatest extent.
Removal of organic matter, such as humic acid, fulvic acid, metabolic secretions of microorganisms, and dissolved animal and plant tissues.
2.3 Catalyst field
Hydrotalcite-type anionic clay is used directly or indirectly as a composite oxide catalyst precursor. It has the advantages of high activity, strong selectivity, uniform distribution of metal cations, and good regeneration repeatability during organic reactions. In addition, due to its special layered structure, roasted or interspersed hydrotalcite-like materials can be used as catalyst carriers. Because of their special structure, the catalytic materials they carry can have better catalytic activity and selectivity. Hydrotalcite materials are used as catalysts, mainly used for alkali catalysis and redox catalysis.
2.4 Pharmaceutical field
The alkalinity of LDH can be used to adjust the pH value of gastric juice, which can prevent and treat some diseases caused by excessive gastric acid. Utilizing the exchangeability of LDH anions, some drug molecules can be introduced into the interlayer and the release rate and location of the drug in the body can be adjusted. It has excellent biocompatibility, anion exchange capacity, high drug loading efficiency, etc. It also has extensive application value as a drug carrier in the pharmaceutical industry.
write at the end
From a market perspective, domestic manufacturers Dandong Songyuan (A-1CPvc, HT-3Pvc), Chenghe Technology (Ac-320, Az-128, Ac-207), Hengshui Shijing (sK-10, sK-20) , Shandong Wanxinna (smoke suppressant, etc.), foreign manufacturers Japan Kyowa Chemical, Japan Tan Chemical, Germany Clariant, South Korea Danshi, etc. At present, binary hydrotalcite technology is relatively mature, especially magnesium-aluminum hydrotalcite, and the market competition is fierce. With the different downstream pursuits of synthetic hydrotalcite performance, ternary or multi-component hydrotalcites have appeared on the market. At present, Chenghe Technology’s Az-128 is a rare ternary synthetic hydrotalcite product in China.
