Abstract
The present invention discloses a flower-shaped magnesium oxide nanostructured material and a preparation method and application thereof. The flower-shaped magnesium oxide nanostructured material provided by the present invention is prepared according to a method comprising the following steps: adopting a microwave-assisted solvothermal method, using cheap inorganic salts and urea as raw materials, ethanol as a solvent, obtaining a precursor in one step without a template, and then calcining in air to obtain the precursor. The preparation method of the flower-shaped magnesium oxide nanostructured material of the present invention is simple, the raw material cost is low, it is safe and non-toxic, easy to industrialize, and has excellent heavy metal ion adsorption performance, and is expected to be used as a water treatment adsorbent, and has broad application value.
Description
A flower-shaped magnesium oxide nanostructured material and a preparation method and application thereof
Technical field
The present invention relates to a flower-shaped magnesium oxide nanostructured material and a preparation method and application thereof.
Background technology
The flower-shaped magnesium oxide nanostructured material has broad application prospects in the fields of adsorption and catalysis because it has the advantages of large specific surface area at nanoscale, high activity, excellent mechanical properties at micrometer scale, and easy practical operation. Flower-like magnesium oxide nanostructured materials are usually prepared by adding surfactants such as PVP (polyvinyl pyrrolidone) and CTAB (hexadecyl ammonium bromide) using the “ethylene glycol-mediated method”. However, this preparation method has high raw material costs, long preparation time, and often requires the removal of the template agent later, and the process is relatively complicated. Microwave-assisted hydrothermal/solvothermal method has fast and uniform heating speed, short time, and high efficiency, and is widely used in the preparation of flower-like nanostructured materials, but there is no report on the preparation of flower-like magnesium oxide nanostructured materials using this method.
The surface of magnesium oxide carries a certain amount of negative charge and has good adsorption capacity for cations, especially for lead ions and cadmium ions. In many areas of my country, the lead ions and cadmium ions in groundwater are seriously exceeded due to natural and industrial emissions, which seriously affects people’s lives and health. The rated concentrations of lead ions and cadmium ions in drinking water stipulated by the World Health Organization (WHO) are 10μg/L and 5μg/L, respectively. The adsorption capacity of the adsorbent materials reported so far is limited, and the further development of new high-efficiency water treatment adsorbents has great practical significance.
Invention content
The purpose of the present invention is to provide a flower-shaped magnesium oxide nanostructured material and a preparation method thereof that can be used for adsorption and removal of heavy metal pollutants in water.
The flower-shaped magnesium oxide nanostructured material provided by the present invention is prepared according to a method comprising the following steps: using soluble cheap magnesium salt and urea as raw materials, ethanol as solvent, and using a one-step microwave-assisted solvothermal method to react magnesium salt and urea under microwave heating conditions to obtain a flower-shaped magnesium oxide nanostructured material precursor; and then calcining in air to obtain a pure phase flower-shaped magnesium oxide nanostructured material.
In the above method, the soluble cheap magnesium salt may be magnesium chloride, magnesium nitrate or magnesium acetate.
In the above method, the solvent may be anhydrous ethanol or industrial ethanol.
In the above method, the ratio of the magnesium salt, urea and ethanol may be (1-1.3) g: 0.6 g: 100 ml.
In the above method, the reaction temperature of the reaction may be 140-170°C and the reaction time may be 30-120 minutes.
In the above method, the calcination temperature may be 400-500°C and the calcination time may be 2-4 hours.
Another object of the present invention is to provide an application of the above flower-like magnesium oxide nanostructured material.
The application provided by the present invention is the application of the flower-like magnesium oxide nanostructured material in adsorbing heavy metal ions in water. The heavy metal ions are preferably lead ions and cadmium ions.
The present invention adopts microwave-assisted solvothermal method, uses cheap magnesium salt and urea as raw materials, ethanol as solvent, and prepares flower-like magnesium oxide nanostructured material precursor without template. By calcining in air, pure phase flower-like magnesium oxide nanostructured material is obtained; the preparation method is simple, low-cost, pollution-free, and easy to industrialize; the prepared flower-like magnesium oxide nanostructured material has a large specific surface area (40-70m2/g), which is used to adsorb lead ions or cadmium ions in water, with significant effect, high adsorption capacity (>1500mg/g), and the adsorption capacity is significantly higher than the currently used activated carbon or activated alumina adsorbent.
Specific implementation method
The method of the present invention is described below by specific examples, but the present invention is not limited thereto.
The experimental methods described in the following examples are conventional methods unless otherwise specified; the reagents and materials, unless otherwise specified, can be obtained from commercial channels.
In the following examples, JEOL-6701F scanning electron microscope (SEM) and JEOL JEM-1011 transmission electron microscope (TEM) were used to characterize the morphology; and Rigaku D/max-γB powder X-ray diffraction (XRD) instrument was used to analyze the phase of the sample. Cu target Kα, wavelength λ = 0.154056nm, rated power 16kW, tube voltage 40kV, tube current 200mA, scanning speed 8°/min were used. Quantachrome Autosorb-1 specific surface area and pore distribution analyzer was used to characterize its pore structure. Inductively coupled plasma-atomic emission spectroscopy (ICP-AES) was used to detect the concentration of lead ions or cadmium ions in water, using Shimadzu ICPE-9000 plasma emission spectrometer, and the test conditions were: high-frequency output power 1.20KW, cooling gas flow 0.6L/min, plasma gas flow 10L/min, carrier gas flow 0.7L/min, exposure time 30 seconds.
Example 1. Preparation of flower-like magnesium oxide nanostructured material (magnesium salt is magnesium nitrate)
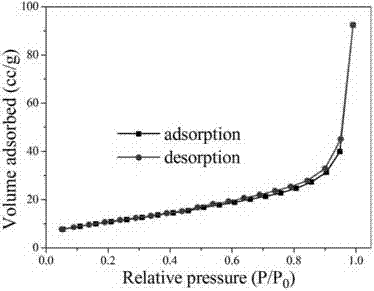
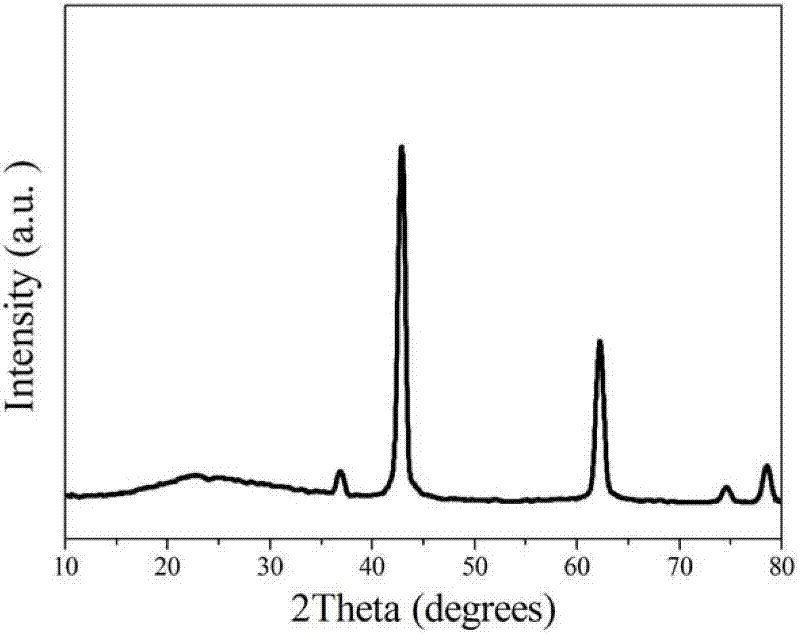
1.3g magnesium nitrate (purchased from Sinopharm Group Co., Ltd.) and 0.6g urea were weighed and dissolved in 100mL anhydrous ethanol. After ultrasonic dissolution, they were placed in a microwave reactor, heated to 150°C within 3 minutes, and then kept for 30 minutes to obtain a flower-like magnesium oxide nanostructured material precursor. After calcination at 400°C in air for 4 hours, a white powder was obtained, which is a flower-like magnesium oxide nanostructured material. X-ray powder diffraction shows that its physical phase is pure magnesium oxide (as shown in Figure 1); SEM (as shown in Figure 2) and TEM (as shown in Figure 3) characterize the morphology of the product, and it can be seen that the product is a typical flower-like shape formed by twisting nanosheets together, with a size of about 1μm. The nitrogen adsorption and desorption results show (as shown in Figure 4) that its specific surface area is 70m2/g.
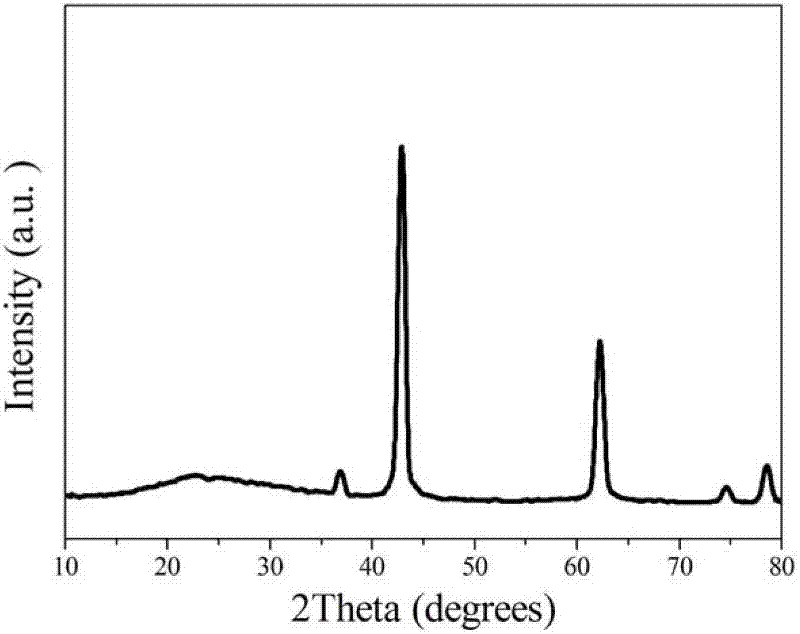

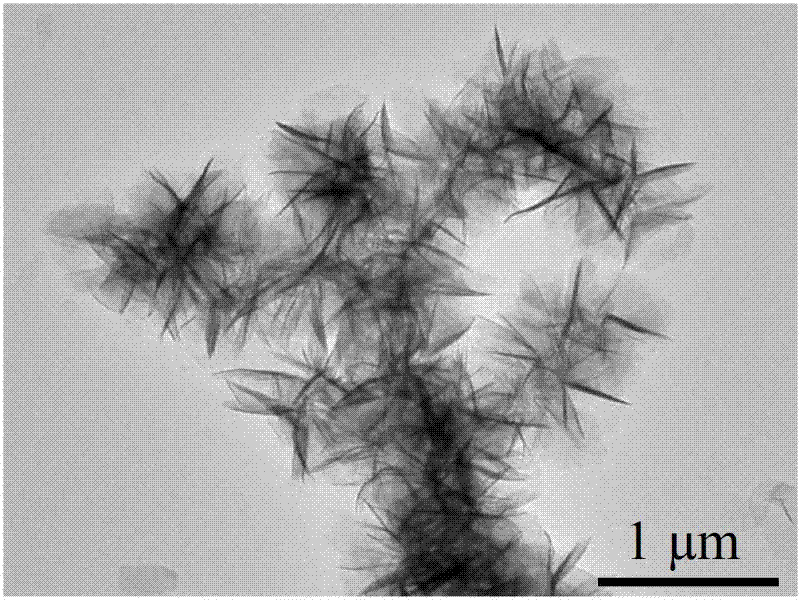
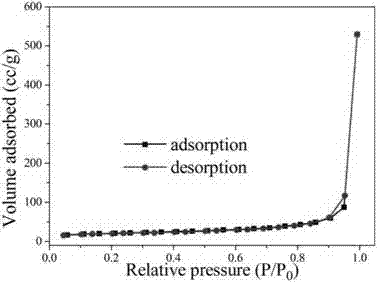
Example 2, preparation of flower-like magnesium oxide nanostructured material (magnesium salt is magnesium chloride)
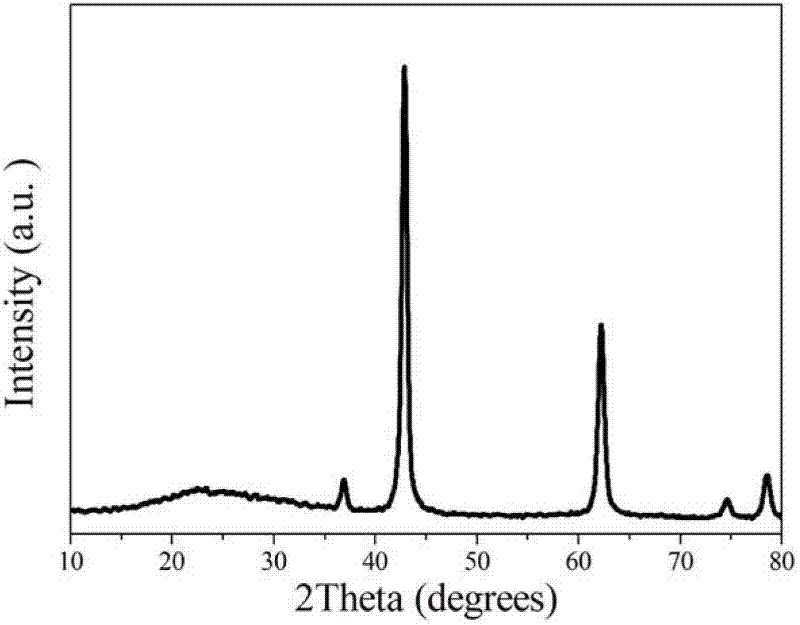
Weigh 1g magnesium chloride (purchased from Sinopharm Group Co., Ltd.) and 0.6g urea and dissolve them in 100mL anhydrous ethanol. After ultrasonic dissolution, place them in a microwave reactor, heat to 140°C within 3 minutes, and then keep it for 120 minutes to obtain a flower-like magnesium oxide nanostructured material precursor. After calcining at 450°C in air for 3 hours, a white powder is obtained, which is a flower-like magnesium oxide nanostructured material. The X-ray powder diffraction spectrum is the same as that of Example 1 (as shown in Figure 5); SEM (as shown in Figure 6) characterizes its morphology, and it can be seen that it is also a flower-like structure assembled by twisted nanosheets, with a size of about 2.5μm. The nitrogen adsorption and desorption results show (as shown in Figure 7) that its specific surface area is 40m2/g.

Example 3, preparation of flower-like magnesium oxide nanostructured material (magnesium salt is magnesium acetate)
Weigh 1g magnesium acetate (purchased from Sinopharm Group Co., Ltd.) and 0.6g urea and dissolve them in 100mL anhydrous ethanol. After ultrasonic dissolution, place them in a microwave reactor, heat them to 170°C within 3 minutes, and then keep them for 30 minutes to obtain a flower-like magnesium oxide nanostructured material precursor. After calcining at 500°C in air for 2 hours, a white powder is obtained, which is a flower-like magnesium oxide nanostructured material. X-ray powder diffraction (as shown in Figure 8) shows that the obtained material is also pure phase magnesium oxide; SEM (as shown in Figure 9) characterizes its morphology, and it can be seen that it is also a flower-like structure assembled by twisted nanosheets, with a size of about 2.5μm.

Example 4, preparation of flower-like magnesium oxide nanostructured material (solvent is industrial ethanol)
The experimental steps are basically the same as those in Example 1, except that the anhydrous ethanol used in Example 1 is replaced by industrial ethanol. The SEM image (as shown in Figure 10) clearly shows its flower-like structure, which is about 3μm in size; the X-ray powder diffraction spectrum is the same as that of Example 1 (as shown in Figure 11).
Example 5, lead adsorption capacity test
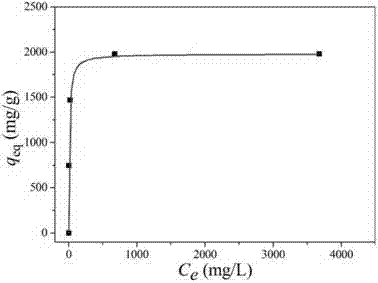
Weigh 20mg of the flower-like magnesium oxide nanostructured material obtained in Example 1 respectively, place it in 50mL lead ion solution of different concentrations, stir at room temperature for 12 hours and then centrifuge, use inductively coupled plasma-atomic emission spectrometer to analyze the upper solution, and obtain the lead ion concentration when the adsorption reaches equilibrium. Combined with the lead ion concentration in the solution before adsorption, the adsorption amount of lead ions in the corresponding solution by this material is calculated. Finally, Langmuir fitting is performed based on these data to obtain the adsorption isotherm curve of lead ions of this material. As shown in Figure 12, the saturated adsorption capacity of lead ions of the flower-like magnesium oxide nanostructured material reaches 1980mg/g.
Example 6, test of adsorption capacity of cadmium
Weigh 20 mg of the flower-like magnesium oxide nanostructured material obtained in Example 1, place it in 50 mL of cadmium ion solution of different concentrations, stir at room temperature for 12 hours and then centrifuge, analyze the upper solution by inductively coupled plasma-atomic emission spectrometer, obtain the lead ion concentration when adsorption reaches equilibrium, and calculate it in combination with the lead ion concentration in the solution before adsorption to obtain the adsorption amount of lead ions in the corresponding solution by this material. Finally, Langmuir fitting is performed based on these data to obtain the adsorption isotherm curve of lead ions by this material. As shown in Figure 13, the saturated adsorption capacity of cadmium ions by the flower-like magnesium oxide nanostructured material reaches 1575 mg/g.
