As a common aqueous magnesium-rich carbonate mineral, hydromagnesite [Mg5(CO3)4(OH)2·4H2O] can be used as a high-quality mineral-based flame retardant filler for polymer flame retardant applications. Studies have shown that hydromagnesite is mainly endowed in carbonate-type salt lakes and Quaternary lake-phase strata, and has also been found in the lake-phase deposits of the Jezero Crater of Mars, and the Mg/Ca value of the lake water is regarded as a key condition for hydromagnesite mineralization.
The conventional view is that the formation of lake-phase hydromagnesite is mainly associated with the weathering of ultramafic rocks to provide Mg-rich source recharge, but the Mg/Ca values of surface river water and groundwater associated with the weathering of ultramafic rocks seldom reach a threshold sufficient to allow direct precipitation of hydromagnesite from salt lakes (Fig. 1a). Therefore, identifying the geochemical cycling process of Mg in the salt lake system is a key component in deciphering the mineralization process of hydromagnesite.
Fig. 1 Chemical compositions of Dujaili Salt Lake water, recharge water and hydromagnesite: (a) Mg/Ca values (edge density plots for global river water and carbonate-type salt lakes); (b) Sr isotope values; and (c) Mg isotope values (edge histograms for reported δ26Mg values of hydromagnesite)
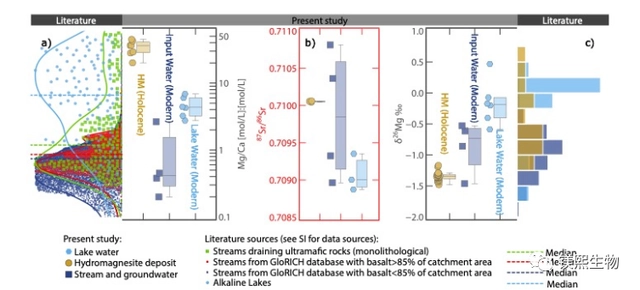
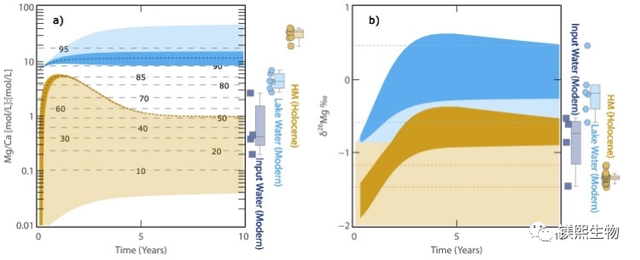
To address the above key scientific issues, the Institute of Mineral Resources of the China Geological Survey (hereafter referred to as “IGR”) and its collaborators systematically carried out Mg isotope studies of saline lake water, river water, groundwater, and hydromagnesite in the Dujiali Salt Lake of Tibet on the example of the Qinghai-Tibetan Plateau Salt Lake Field Observatory of the Ministry of Natural Resources of China (Fig. 1c). 1c). Using Mg isotope analysis and combining with the water chemistry simulation software Phreeqc, the present work was the first to establish a semi-quantitative hydrochemical evolution model for the changes of Mg/Ca values and δ26Mg in the Dujari Salt Lake under evaporation conditions (Fig. 2), and explored the geochemical cycling process of Mg in the salt lake system.
The results show that under strong evaporation conditions, under the evolutionary process of Lake Dujaili, the first saturated precipitation and precipitation out of the Chinese headstone leads to an increase in the Mg/Ca value and alkalinity of the lake water, which in turn drives the saturation of the hydromagnesite and precipitation and precipitation out of the water, and the Mg isotopes provide good parameter qualification for the model. The model results are further corroborated by the fact that in the vast majority of lake-phase hydromagnesite deposits globally, hydromagnesite is usually coeval or alternately deposited with aragonite. In summary, the present work proposes that although surface river water or groundwater associated with the weathering of Mg-rich rocks provides an important source of Mg to salt lakes, however, aragonite precipitation under evaporative conditions may be an important mineralization process driving the deposition of aqueous magnesite in salt lakes.
Fig. 2 Results of hydrochemical evolution modeling of Dujaili Salt Lake (a) Mg/Ca values of lake water (blue) and hydromagnesite (brown); (b) δ26Mg values of lake water (blue) and hydromagnesite (brown)