Hebei Messi Biology Co., Ltd. stated that in recent years, there have been many literature reports on the use of magnesium oxide as a catalyst and as a carrier in various organic reactions. Through electron spin resonance (ESR) characterization, it was found that the catalytic performance of magnesium oxide is caused by the presence of lattice defects and rich “acid-base active sites” on its surface.
The study of crystal structure is essential to understanding the unique properties of magnesium oxide. When studying nanocrystals, the number of surface atoms must be considered. The surface chemical activity of oxides is not only closely related to Lewis acids and bases and Bronsted acids and bases, but also to the combination between metal cations, oxide anions and -OH. The number of atoms on its surface is large, but the number of atoms in the lattice is limited, so nanoparticles have many so-called lattice defects. Point defects account for the main part of lattice defects. Point defects are caused by the lack of a single atom in the lattice. Or ions, point defects cause atoms or ions to be displaced due to polarization, resulting in the creation of negatively charged cation “gaps”, which in turn leads to the transfer of adjacent anions. Therefore, there are many active sites on the surface of magnesium oxide that are conducive to catalytic reactions.
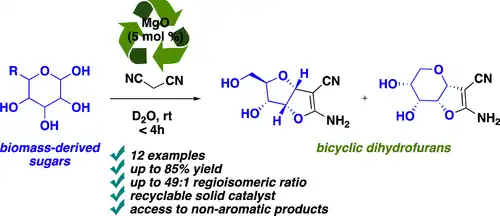
The main advantages of magnesium oxide as a catalyst for multicomponent condensation reactions are the simplicity of synthesis, thermal stability, the possibility of recovery and reuse from the reaction mixture, as well as its high activity and reaction yield. An example of the use of basic magnesium oxide to catalyze organic condensation reactions is the synthesis of tetrahydropyrene and 3,4-hydropypyrene reported by Sheibani. Heterocyclic pyrene is an aromatic aldehyde in a system containing α-hydroxyl or α-amino acid and malononitrile. Synthesized by reaction. Expanding the synthesis of heterocyclic compounds that occupy an important position in biopharmaceuticals is currently the focus of the scientific community. Pyran and its derivatives are compounds with very important value in anti-thrombosis, relaxation and anti-tumor. Considering magnesium oxide The high catalytic activity in the Knoevenagel condensation reaction and the low cost of its production and use make this research on the synthesis of pyrans catalyzed by magnesium oxide more attractive than the former.

