The crystallization processes of hydrated magnesium carbonate (Mg5(CO3)4(OH)2-4H2O) and magnesium carbonate (MgCO3-3H2O) involve complex chemical bonding changes. Here are some key points based on chemical bonding analysis:
Crystal structure :
Mg5(CO3)4(OH)2-4H2O: This crystal has a hexagonal lamellar crystallization habit. In its structure, each magnesium ion (Mg) is bonded to four carbonate ions (CO32-) and four hydroxyl ions (OH–) to form an octahedral structure. In addition, each carbonate ion is also connected to two other magnesium ions by covalent bonds to form a layered structure.
MgCO3-3H2O : This crystal has a hexagonal columnar structure. In its structure, each magnesium ion is bonded to three carbonate ions and three water molecules to form a tetrahedral structure. The carbonate ions are connected to each other by covalent bonds to form a chain structure.
Number and strength of chemical bonds:
Mg5(CO3)4(OH)2-4H2O : In the hexagonal lamellar structure, each crystal face contains a higher number of chemical bonds, especially the (001) crystal face, the number and strength of which have an important influence on the growth rate and morphology of the crystal. The crystal growth behavior can be predicted by calculating the number of chemical bonds in different crystal faces.
MgCO3-3H2O : In the hexagonal columnar structure, the (001) crystal face is also the most important growth face, and the number and strength of its chemical bonds directly affect the growth direction and morphology of the crystal.
Experiment and Theory:
Experimental results : It can be observed experimentally that the morphology of magnesium carbonate trihydrate crystals and the aspect ratio of whisker products can be controlled by adjusting the reaction conditions (e.g., initial concentrations of Mg2+ and NH4HCO3, reaction temperature and time). For example, whiskers of magnesium carbonate trihydrate with a high aspect ratio can be synthesized under specific conditions.
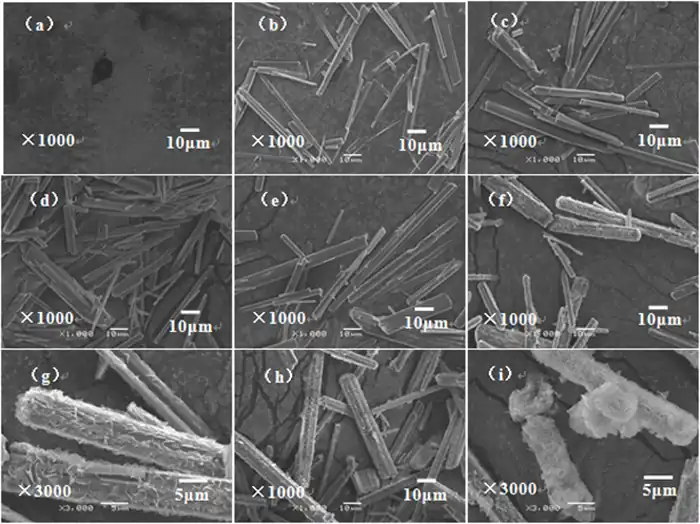
Theoretical calculations : Based on crystallographic structure and chemical bonding theories, it is possible to quantitatively analyze the crystallization behavior of magnesium carbonate trihydrate and to predict its ideal morphology.1 These theoretical calculations are in agreement with the results of actual experiments, which proves the validity of the theoretical approach.
Applications :
Crystal growth optimization : The intrinsic improvement of single-crystal growth can be achieved by adjusting the bonding mode of the constituent atoms or ions in a thermodynamic sense. This provides a broader scope for optimizing the experimental strategy from a kinetic point of view.
In summary, the crystallization process of hydrated magnesium carbonate is controlled by the number and strength of chemical bonds, and the crystal growth process can be optimized through theoretical calculations and experimental verification by effectively predicting and controlling the crystal growth behavior.

