Magnesium hydroxide is an important inorganic chemical with the appearance of white amorphous powder, which occupies an important position in modern economic development. It plays an important role in environmental protection (such as flue gas desulfurization, wastewater neutralization, coal sulfur fixation, heavy metal removal, etc.), material processing (such as fine ceramics, coatings, flame retardant, electronic materials), health care, food processing, and so on.
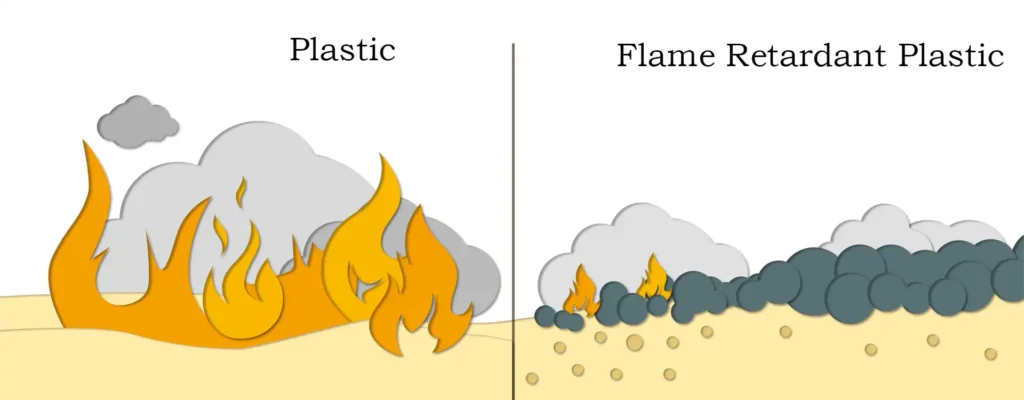
Relative to organic flame retardants, inorganic flame retardants have obvious advantages – in case of fire will not produce a large number of harmful and toxic gases, providing convenient conditions for people to escape. Moreover, magnesium hydroxide has the advantages of non-toxicity, smoke suppression, high temperature resistance, low cost and so on, which is a kind of green flame retardant. Next to give you an introduction to its flame retardant mechanism and characteristics:
- Heat-absorbing effect: magnesium hydroxide will undergo a dehydration reaction when heated, generating water vapor and magnesium oxide. This reaction is a heat-absorbing reaction, which can absorb a large amount of heat, thus reducing the temperature of the surface of the burning material and playing a flame retardant role.
- Dilution of oxygen concentration: the water vapor generated by the thermal decomposition of magnesium hydroxide can dilute the oxygen concentration in the burning area and hinder the combustion process.
- Formation of carbonized layer: magnesium hydroxide decomposition of magnesium oxide has good refractory properties, can form a layer of carbonized layer on the surface of the combustible, this layer of carbonized layer can isolate the air, preventing the combustible and the outside world of the contact with oxygen, so as to slow down the combustion process.
- Inhibition of combustion reaction: magnesium hydroxide decomposition of magnesium oxide particles can be generated with the free radicals generated in the combustion process, inhibit the combustion reaction, reduce the combustion rate and flame temperature.
Application areas of magnesium hydroxide flame retardant
Magnesium hydroxide flame retardant is widely used in many fields:
- Plastics industry: used in polyolefin plastics (such as polyethylene, polypropylene, etc.) and polyurethane and other plastics, significantly improve the flame retardant properties of plastics.
- Rubber industry: used in flame retardant rubber sheet, flame retardant hose and other rubber products.
- Textile industry: Used in the flame retardant treatment of textiles to improve the flame retardant properties of textiles.
- Building materials industry: used in flame-retardant coatings, flame-retardant wood and other building materials to improve the flame-retardant properties of building materials.
- Electronic and electrical industry: used in the flame retardant treatment of wire and cable, electronic components and other electronic and electrical products to improve their safety.
Advantages and disadvantages of magnesium hydroxide flame retardant
Advantages:
- Non-toxic, smoke suppression, high temperature resistance, low cost, is a green flame retardant.
- It will not produce harmful and toxic gases in fire, which is favorable for people to escape.
Disadvantages:
- Requires a large additive amount to achieve the desired flame retardant effect, which may affect the mechanical properties and processing performance of the material.
- Poor compatibility with the matrix, easy to agglomerate, affecting dispersion and compatibility.
Surface modification methods of magnesium hydroxide flame retardants
In order to improve the dispersion and compatibility of magnesium hydroxide, the following surface modification methods are usually used:
- Chemical modification: using coupling agent, compound modifier and surfactant for modification.
- Microencapsulation modification: to improve the thermal stability and interfacial viscosity of magnesium hydroxide, and improve the mechanical properties.
- Surface grafting modification: graft the modifier on the polymer surface to form a macromolecular modifier to improve the surface properties of polymer materials.
Through these methods, the efficiency and cost-effectiveness of magnesium hydroxide flame retardants can be effectively improved, so that they can play a better role in various applications.

