Messi Biology stated that in recent years, fire accidents caused by polymers have attracted great attention from all walks of life, and the research and development of flame-retardant polymers has become a hot topic. At present, the organic flame retardants used in the market have hazards such as large smoke production and toxic smoke, which have been restricted by laws in Europe, the United States, Japan and my country. Therefore, inorganic flame retardants have received great attention.
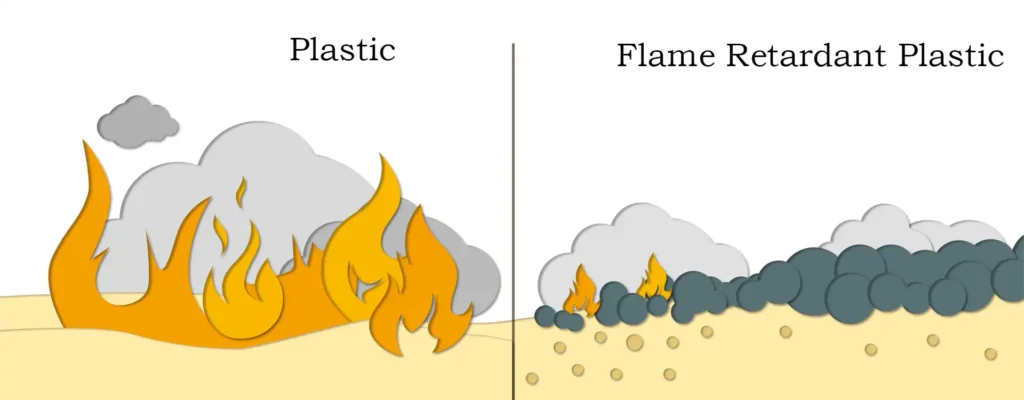
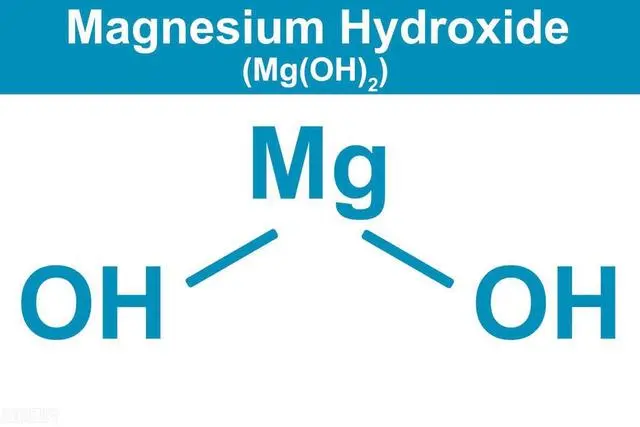
Magnesium hydroxide flame retardant has a high decomposition temperature (340℃~450℃), and the thermal decomposition products are MgO and H2O. It does not release any toxic and harmful substances and has no harm to the environment and human health. Therefore, magnesium hydroxide flame retardant has become one of the most concerned inorganic flame retardants and has broad application prospects.
Flame retardant mechanism of magnesium hydroxide
Magnesium hydroxide has a special layered structure, which makes it have excellent thixotropy and low surface energy, and has good flame retardant and smoke elimination effects on plastics. Magnesium hydroxide begins to decompose into magnesium oxide and water at 340℃. The temperature can reach 490℃ when it is completely decomposed, and it absorbs a lot of heat energy during decomposition. The specific flame retardant mechanism is as follows:
(1) Magnesium hydroxide has a large heat capacity. When it is thermally decomposed, it absorbs a large amount of heat and releases a large amount of water vapor, which not only reduces the temperature of the material surface, but also reduces the generation of flammable small molecules.
(2) The large amount of water vapor generated by thermal decomposition can also cover the surface of the material, reduce the oxygen concentration in the air on the combustion surface, and thus hinder the combustion of the material.
(3) Magnesium oxide generated by the thermal decomposition of magnesium hydroxide is a good refractory material. It can not only cover the surface of the material, but also promote the carbonization of the polymer material, forming a carbonized layer to block the entry of heat and air, thereby effectively preventing combustion.
(4) Magnesium hydroxide has the function of a redox reaction catalyst, which can promote the conversion of CO into CO2 during combustion; the magnesium oxide generated by decomposition can neutralize SO2, CO2 and NO2 generated during combustion, thereby reducing the release of toxic and harmful gases.
Preparation of magnesium hydroxide flame retardant
01 Physical pulverization method
The physical pulverization method is a method of using mechanical or ultrasonic methods to pulverize and ultrafine natural minerals (mostly brucite) to obtain magnesium hydroxide within the required particle size range. Although the physical pulverization method is simple in process and low in cost, the prepared magnesium hydroxide has low purity and uneven particle size distribution. It is usually necessary to use a special grinding method or add a grinding aid (or dispersant) during the grinding process to obtain high-quality magnesium hydroxide. Therefore, its application and development in industry are greatly restricted.
02 Chemical solid phase method
The solid phase method for preparing magnesium hydroxide is a process in which solid metal salts and metal hydroxides are mixed in a certain proportion, ground and calcined, and a solid phase reaction occurs to obtain a magnesium hydroxide product. This method has the characteristics of simple process and low cost, but it also has defects such as low product purity, easy agglomeration, and poor dispersion performance. It is rarely used in actual large-scale industrial production.
03 Chemical gas phase method
The gas phase method for preparing magnesium hydroxide is to use ammonia as a precipitant and directly pass ammonia into a solution containing Mg2+ to prepare magnesium hydroxide. The quality of magnesium hydroxide prepared by the gas phase method is affected by factors such as ammonia flow rate, stirring intensity and reaction temperature. In the process of preparing magnesium hydroxide flame retardant by gas phase method, the ammonia concentration is stable, and the product has the advantages of high purity, uniform particle size and good dispersion performance. At the same time, no water is introduced during the introduction of ammonia, and the obtained magnesium hydroxide slurry has high concentration, small footprint in the production process, and high unit equipment yield. However, it has high requirements for equipment and technology, and is also prone to ammonia diffusion and environmental pollution.
04 Chemical liquid phase method
The liquid phase method for preparing magnesium hydroxide is to use magnesium salt as the main raw material, react it with an alkaline substance containing hydroxide ions (OH-) to generate magnesium hydroxide precipitate, and then wash and dry to obtain the product. The liquid phase method can be divided into direct precipitation method, solvent thermal and hydrothermal method, precipitation-azeotropic distillation method, ultrasonic chemical method and microwave assisted method.
(1) Direct precipitation method
Direct precipitation method is also called alkali method, which is a method of directly reacting magnesium solution with alkaline precipitant or precipitant precursor to generate magnesium hydroxide. According to the type of precipitant, it can be divided into lime method, ammonia method, sodium hydroxide method and calcium hydroxide method. The direct precipitation method is simple and easy to operate, has low requirements for equipment and technology, and is not prone to producing impurities. However, its reaction conditions affect the performance of the final product. The raw material concentration, reaction process, reaction time, temperature, stirring rate, etc. of magnesium hydroxide preparation are all the focus of current research.
(2) Solvothermal and hydrothermal method
The solvent thermal and hydrothermal method is a chemical synthesis method that is easy to control the size and dispersion of magnesium hydroxide. This method changes the properties of magnesium hydroxide under high temperature and high pressure, and the magnesium salt in the raw material reacts and crystallizes with the alkaline substance to form magnesium hydroxide with more uniform particles and higher dispersion. Current research on the solvent thermal and hydrothermal method mainly focuses on improving the performance of magnesium hydroxide products, such as adding different types of organic solvents or additives, and reasonably adjusting the chemical reaction time and reaction temperature.
(3) Precipitation-azeotropic distillation method
The precipitation-azeotropic distillation method can improve the agglomeration phenomenon in the conventional preparation process of magnesium hydroxide. The principle is that the particles of the general precipitate are filled with water molecules. Direct drying easily causes the particles to form hard agglomerates under the action of capillary pressure. The azeotropic distillation method uses organic matter such as alcohols and water to form azeotropes at a certain temperature, thereby removing the water in the magnesium hydroxide colloid, thereby improving its dispersibility and obtaining a product with good dispersibility.
(4) Ultrasonic chemical method and microwave-assisted method
Ultrasonic chemical method and microwave-assisted method are both new magnesium hydroxide flame retardant preparation processes. Ultrasonic chemical method is a chemical reaction triggered under extreme conditions. It mainly relies on ultrasound to trigger the formation and collapse of microbubbles, so that active sites are generated under high temperature and high pressure, thereby enhancing the chemical reaction rate and ensuring that the morphology of magnesium hydroxide particles is more uniform and unified. The research on this method mainly focuses on ultrasonic power, product performance and other aspects, which can enhance the comprehensive advantages of ultrasonic chemical method for preparing magnesium hydroxide flame retardants. Ultrasonic chemical method does not require pressure control during the reaction process, the overall reaction speed is faster, the reaction temperature is relatively low, and the process control is more advantageous.
Using microwave technology to prepare magnesium hydroxide consumes relatively less energy and does not cause serious pollution to the environment. At the same time, the microwave-assisted method can effectively shorten the chemical reaction time of magnesium hydroxide by heating, so that a more uniform high-temperature state can be formed inside the sample solution. The microwave-assisted method can be used in combination with the hydrothermal method to further explore new methods and deep application value of magnesium hydroxide flame retardant preparation.
Application requirements of magnesium hydroxide flame retardant materials
As a flame retardant, magnesium hydroxide has the following requirements:
(1) It must have extremely high purity (Mg(OH)2>93%). Magnesium hydroxide with high purity not only has high flame retardant properties, but also can reduce its addition amount in the material.
(2) Small particle size. The composite materials prepared with micro-nano magnesium hydroxide are far superior to micron-level magnesium hydroxide in all aspects of performance (including flame retardant effect, smoke elimination and mechanical properties, etc.).
(3) Low surface polarity. When the surface polarity of magnesium hydroxide decreases, the degree of agglomeration will decrease, and the dispersibility and compatibility will increase. It can be added to polymers as a flame retardant material to have good compatibility with polymers and reduce the impact on the mechanical properties of the material.
Reasons for modification of magnesium hydroxide flame retardant materials
At present, most of the magnesium hydroxide flame retardants produced on the market are micron-sized (d>5μm), with a wide particle size distribution, and require a large amount of filling in the application; in addition, the prepared magnesium hydroxide products are easy to agglomerate, highly hydrophobic, and incompatible with high molecular polymers. In practical applications, magnesium hydroxide has caused serious damage to the mechanical properties of high molecular polymer materials, greatly limiting the application of magnesium hydroxide flame retardants.
Obtaining magnesium hydroxide flame retardants with low surface polarity, strong hydrophilicity, small particle size and narrow distribution, and good compatibility with high molecular polymers through certain physical and chemical methods has become a hot topic for current scientific and technological workers.
On the one hand, the use of organic functional groups to modify the surface of magnesium hydroxide can reduce the polarity of the surface of magnesium hydroxide and improve its compatibility with high molecular polymers; the filling amount of ultrafine magnesium hydroxide at the micro-nano level is low, and the composite materials prepared using it have good performance.
On the other hand, the flame retardant performance of micro-nano magnesium hydroxide of the same quality is several times higher than that of micron-sized magnesium hydroxide, and the impact on the performance of polymer high molecular materials is also low. Micro-nano magnesium hydroxide with low polarity can be evenly dispersed in high molecular polymer materials, so that the flame retardant and mechanical properties of the whole material remain consistent. Therefore, surface modification and ultrafineness of magnesium hydroxide flame retardants can solve the shortcomings in the application of magnesium hydroxide flame retardants.
Research on surface modification of magnesium hydroxide
As a flame retardant for high molecular polymers, the most important thing for magnesium hydroxide is to be well compatible with high molecular polymers, achieve uniform dispersion, and ultimately achieve the purpose of flame retardancy. Using specific compounds to modify the surface of magnesium hydroxide can reduce the surface polarity of magnesium hydroxide, make its surface hydrophobic, and improve the compatibility of magnesium hydroxide with polymers.
The surface chemical modification of magnesium hydroxide is to selectively adsorb or specifically adsorb or react the functional groups in organic molecules or inorganic gel molecules on the surface of magnesium hydroxide powder by chemical methods, thereby coating the surface of the particles, making the surface of the particles organic or changing the polarity, and finally achieving surface modification. Commonly used surface modifiers mainly include silane coupling agents, titanates, aluminates coupling agents, higher fatty acids and their derivatives, etc.
The physical modification of magnesium hydroxide usually includes surface high energy modification and surface coating modification. Surface high energy modification mainly modifies Mg(OH)2 by radiation and other methods to change the surface activity of Mg(OH)2. The modification process does not involve chemical reactions. Surface coating modification mainly modifies Mg(OH)2 by dispersants.
Research on ultrafine magnesium hydroxide
Ultrafine is one of the effective methods to enhance the compatibility of magnesium hydroxide with polymers and reduce the filling amount. The preparation process has a decisive influence on the physical and chemical properties of the final morphology of magnesium hydroxide, such as particle size, particle size distribution, and morphology. Changing the preparation process is the most effective way to obtain ultrafine magnesium hydroxide.
Solvent thermal and hydrothermal methods, microwave-assisted methods, and ultrasonic chemical methods have been widely verified in the preparation of micro-nano materials, and there have also been some research reports on the preparation of ultrafine magnesium hydroxide flame retardant materials.
Messi Biology stated that in the past two decades, great progress has been made in the research on magnesium hydroxide modification and ultrafine preparation. The non-toxicity, wide availability and low price of magnesium hydroxide are the biggest advantages of this material as a flame retardant. With the strengthening of environmental protection efforts, the application of magnesium hydroxide in flame retardant materials will continue to increase in the future. The surface modification and ultra-fine preparation of magnesium hydroxide flame retardant are of great significance to its application in the flame retardant material industry.

