1 Introduction
Cobalt is an important metal resource, cobalt is widely used in aerospace, machinery manufacturing, electrical and electronic, chemical and ceramic industries because of its high temperature resistance, corrosion resistance and magnetic properties, is one of the important raw materials for the manufacture of high-temperature alloys, cemented carbides, ceramic pigments, catalysts and batteries [1-3], especially in the current rapid development of electric vehicles and other industries leading to the field of cobalt power batteries on the demand for cobalt. The demand for cobalt in the field of cobalt power battery is soaring. Cobalt resources are mostly associated with copper cobaltite, nickel cobaltite, arsenic cobaltite and pyrite deposits, and there are very few independent cobalt minerals, and the terrestrial resource reserves are relatively small, and the seafloor manganese nodules are an important prospective resource of cobalt [4-7].
In this study, for a low-grade oxidized copper-cobalt ore in Kolwezi, D.R. Congo, copper and cobalt were recovered by sulfuric acid reduction leaching [8-12], copper and cobalt separation by leach solution extraction, decontamination of extraction residue, cobalt precipitation in the first section and cobalt precipitation in the second section, etc. The effects of different leaching conditions, different decontamination conditions and different cobalt precipitation conditions on the cobalt extraction were examined, which can be used as a reference for the development of similar ores.
2 Raw materials, chemicals and test methods
2.1 Analysis of sample properties
The results of multi-element analysis and mineral composition of the samples are shown in Table 1 and Table 2 respectively.

Table 1 Multi-element analysis of the sample /%
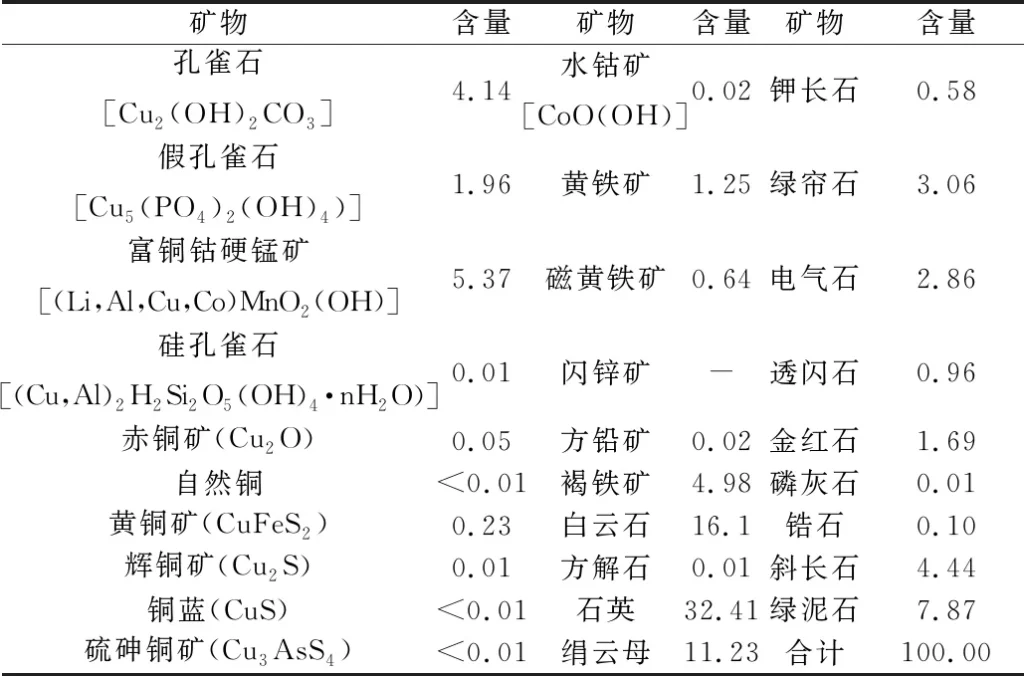
Table 2 Mineral composition and content of the samples /%
From Table 1 and Table 2, it can be seen that the recoverable valuable elements in the samples are copper and cobalt, and the metal minerals are dominated by copper-containing metal oxides and limonite, followed by pyrite and copper sulfides, etc. The samples were analyzed for copper and cobalt. Copper minerals are mainly malachite, pseudomalachite and copper-cobalt-rich stibnite, a small amount of chalcopyrite and chalcopyrite, trace silica malachite, chalcopyrite, copper blue, sulfur-arsenocopperite and natural copper. Cobalt-bearing minerals are mainly copper-cobalt-rich stibnite and a small amount of water cobaltite. In addition to copper and cobalt minerals, other metal minerals are mainly limonite and pyrite, trace sphalerite and galena.
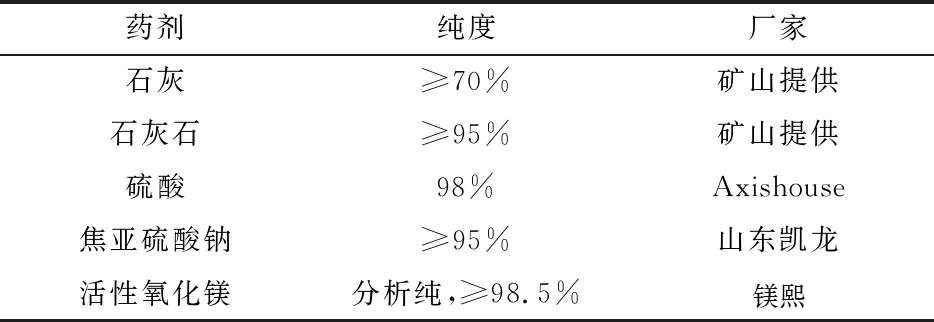
2.2 Agents
The agents used in the test are shown in Table 3, and the test water is tap water.
Table 3 Main test agents
2.3 Test method
Malachite and silica malachite and other copper oxide minerals can react with acid to generate copper ions into solution, trivalent cobalt oxides need to be reduced in acidic conditions for leaching, this test uses sulfuric acid and sodium metabisulfite to leach copper and cobalt, the leach solution using extraction – reverse extraction – electrowinning method to recover copper, cobalt is left in the residual solution, the use of limestone and lime to remove the copper residual solution of copper and iron and other impurities. Limestone and lime are used to remove copper and iron and other impurities in the residual solution, after removing impurities, the solution uses activated magnesium oxide to precipitate cobalt in the first stage to get cobalt hydroxide products, and the residual solution after precipitating cobalt in the first stage is used to precipitate cobalt in the second stage with lime, and the solution after precipitating cobalt in the second stage is returned to the leaching process.
2.3.1 Copper and cobalt leaching
Weighing quantitative fine grinding to -0.074 mm content of 65% of the sample poured into a beaker, according to the slurry mass concentration of 33% to add tap water, turn on the stirring, add sulfuric acid to control the endpoint pH value of 1.5 ~ 1.8, in the process of adding sodium metabisulfite in a different way, the reaction for a certain period of time, take out the filtration, residue and liquid sent to the inspection.
The chemical reaction equation is as follows:
Cu2(OH)2CO3+2H2SO4=2CuSO4+CO2↑+3H2O
CuSiO3-2H2O + H2SO4=
CuSO4 + SiO2-nH2O + (3-n)H2O
4Co(OH)3 + 3H2SO4 + Na2S2O5=
4CoSO4+Na2SO4+9H2O
2.3.2 Removal of impurities from copper extraction residue
After the extraction of copper leaching solution to take a certain amount of extracted liquid poured into a beaker, add a certain amount of limestone emulsion stirring reaction for a certain period of time, then add lime emulsion, continue to react for a certain period of time after filtration, the residue were sent to the test.
The main chemical reaction formula is as follows:
CaCO3+ H2SO4+ H2O= CaSO4-2H2O↓ + CO2↑
Al2(SO4)3+3CaCO3+9H2O= 2Al(OH)3+3CaCO3+9H2O
2Al(OH)3↓ + 3CaSO4-2H2O↓ + 3CO2↑
2FeSO4+O2+SO2= Fe2(SO4)3
Fe2(SO4)3+3CaCO3+9H2O=
2Fe(OH)3↓ +3CaSO4-H2O↓+3CO2↑
MnSO4+2CaCO3+O2+SO2+4H2O=
MnO2↓ +2CaSO4-2H2O↓ +2CO2↑
CaO + H2SO4+H2O= CaSO4-2H2O↓
2.3.3 Cobalt deposition test
Activated magnesium oxide with tap water formulated into a mass concentration of 20% of the emulsion, after the removal of impurity liquid poured into a beaker, stirring state slowly to the solution after the removal of impurity add activated magnesium oxide emulsion, stirring for a certain period of time after the filtration, slag and liquid were sent to the test.
The main chemical reaction formula of cobalt precipitation is as follows:
MgO+CoSO4+H2O=MgSO4+Co(OH)2↓
3CoSO4+MgO +H2O=Co3(OH-SO4)2↓+MgSO4
MnSO4+MgO +H2O= Mn(OH)2↓+MgSO4
MgO+ H2O= Mg(OH)2
3 Test results and discussion
3.1 Copper and cobalt leaching
Test conditions: grinding fineness -74 μm content of 70%, stirring leaching, slurry mass concentration of 33%, adding sulfuric acid to control the endpoint pH in 1.5 ~ 1.8, reaction time of 5 h and then filtered, adding a certain amount of tap water for washing.
3.1.1 Effect of sodium metabisulfite dosage
Sodium metabisulfite was added 30 min after the addition of sulfuric acid, and the effects of different sodium metabisulfite dosages on the leaching rate of copper and cobalt are shown in Table 4.
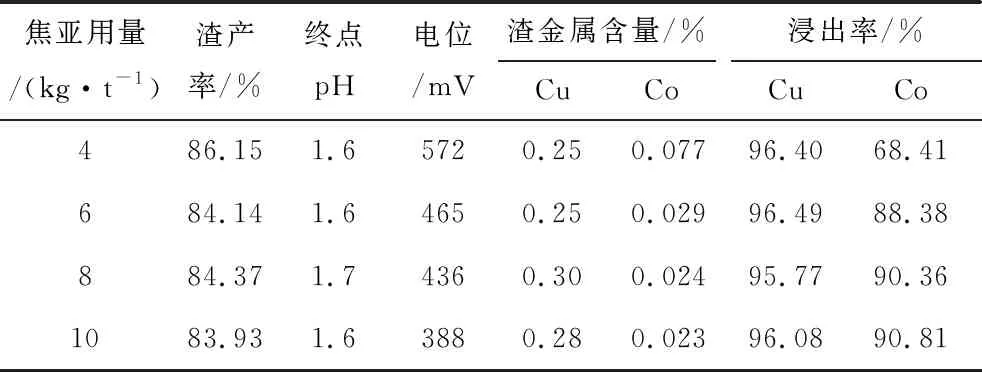
Table 4 Test results of sodium metabisulfite dosage
As can be seen from Table 4, with the increase of sodium metabisulfite dosage, the copper leaching rate does not change much, are above 95%, and the cobalt leaching rate gradually increases, and it is recommended that the dosage of sodium metabisulfite is 8 kg/t ore.
3.1.2 Influence of sodium metabisulfite addition method
Fix the dosage of sodium metabisulfite 8 kg/t, carry out the leaching effect test of Co under different sodium metabisulfite addition methods, the addition methods are divided into the following six: (1) sulfuric acid is added at one time at the beginning of the test; sodium metabisulfite is added at one time at the beginning of the test; (2) sulfuric acid is added at one time at the beginning of the test, and sodium metabisulfite is added at the beginning of the test for 30 min; (3) sulfuric acid is added at one time at the beginning of the test; (4) sulfuric acid is added at one time at the beginning of the test; (5) sulfuric acid is added at the beginning of the test; and sodium metabisulfite is added at the beginning of the test for 30 min. one-time addition at the beginning of the test; sodium metabisulfite was added in two equal additions at 30 and 60 min of the test; (4) sulfuric acid was added in two equal additions at the beginning of the test and at 60 min; sodium metabisulfite was added in two equal additions at the 30th and 90th min of the test; (5) sulfuric acid was added in three equal additions at the beginning of the test, at 30 min and at 60 min, and sodium metabisulfite was added in three equal additions at the 30th and 90th min. Sodium metabisulfite was added in three equal additions, at the 60th, 80th and 100th min of the test; (6) Sulfuric acid was added in three equal additions, at the 30th, 60th and 90th min of the test; and sodium metabisulfite was added in three equal additions, at the beginning of the test, 20th and 40th min of the test, respectively. the results are shown in Table 5.
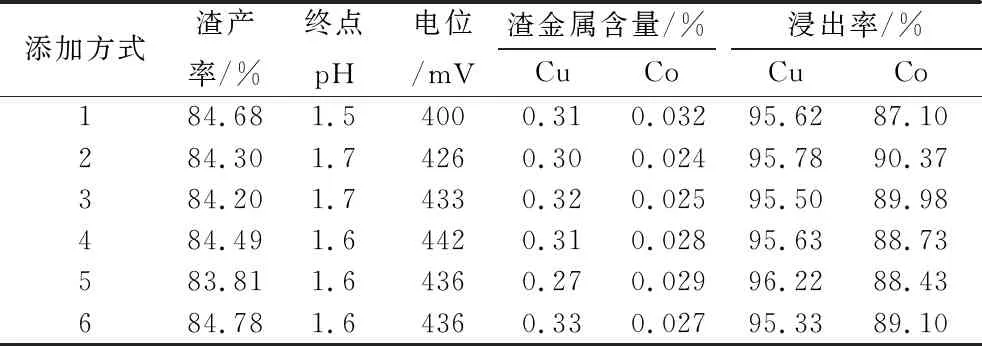
Table 5 Test results of different ways of adding sodium metabisulfite
As can be seen from Table 5, different sodium metabisulfite addition methods have no obvious effect on the leaching rate of Cu, and the leaching rate of Cu reaches more than 95%; the leaching rate of Co in addition method 2 is the highest, reaching 90.37%, which is 3.27% higher than that of sodium metabisulfite and sulfuric acid added at the same time.
3.1.3 Effect of leaching time
Test conditions: the amount of sodium metabisulfite is 8 kg/t, the addition method is the above method (2), to investigate the leaching rate of copper and cobalt under different leaching time, the results are shown in Table 6.
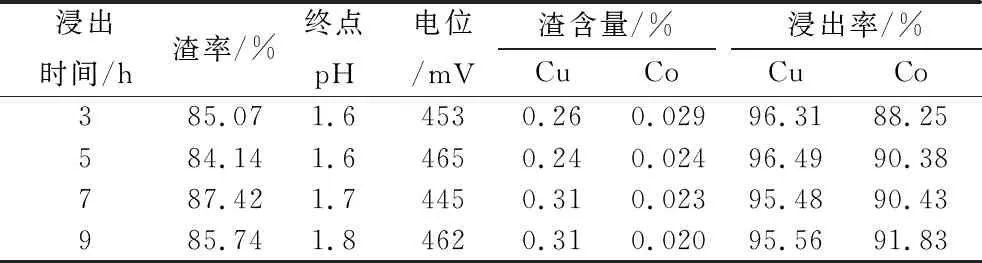
Table 6 Test results of different leaching time
As can be seen from Table 6, with the extension of leaching time, the pH of the leaching slurry increased, the copper leaching rate is not much different, the copper leaching rate is above 95%, the cobalt leaching rate increased slightly, the leaching time of about 5 h is appropriate, the cobalt leaching rate is 90.38%.
3.2 Copper extraction residue removal
After the extraction of copper leachate obtained by the extraction residue multi-element analysis results are shown in Table 7, due to copper, iron and manganese precipitation pH is lower than or similar to the cobalt precipitation pH, in order to ensure that the subsequent cobalt precipitation process to obtain qualified cobalt hydroxide products, the need to remove the extraction of impurities in the residue of copper, iron and manganese and so on.

Table 7 Multi-element analysis of extracted liquid
Limestone price is low, and not easy due to excessive alkali caused by co-precipitation of cobalt, but if all the use of limestone to adjust the pH, pH more than 4.5, limestone is easy to add too much, so the use of limestone and lime with the neutralization of iron precipitation of copper, the first to join the limestone, pH adjusted to 3.5 ~ 4.0, limestone dosage of 20 kg/m3 extracted residual solution, and then continue to add a small amount of lime to adjust pH to 5.5 to 5.5, and then continue to add a small amount of lime, will be Then add a small amount of lime to adjust the pH to 5.5~5.8, lime dosage 2.2 kg/m3 extracted liquid. The test results are shown in Table 8.
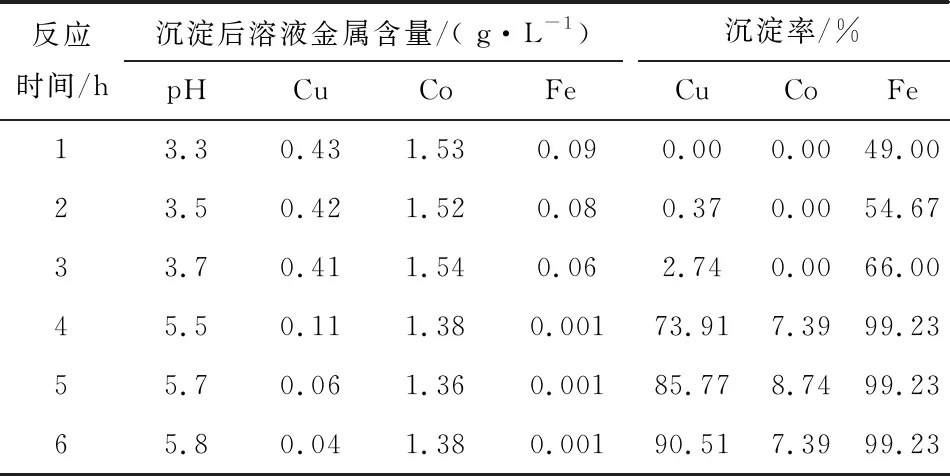
Table 8 Test results of iron and copper precipitation with different reaction times
As can be seen from Table 8, after the limestone is added, as the reaction proceeds, the iron removal rate rises gradually, the copper precipitation rate increases slightly, and cobalt basically does not precipitate; the reaction is about 3 h, the pH value can reach 3.7, at this time, the iron content is 0.06 g/L; after the lime is added, as the reaction proceeds, the copper precipitation rate increases gradually, and the copper content in the solution can be lowered to 0.04 g/L after lime is added for 3 h. The copper precipitation rate is 90.51%, and the iron precipitation rate is 0.04%, and the iron precipitation rate is 90.51%, and the iron precipitation rate is 90.51%. 90.51%, iron precipitation rate of 99.23%, cobalt precipitation rate of 7.39%. The precipitation slag is returned to the leaching process to recover copper and cobalt, iron is partially leached, and the unleached part is discharged together with the leaching slag of the original ore.
3.3 Cobalt precipitation
Magnesium oxide is chosen as the cobalt precipitation agent, compared with lime, the precipitation product obtained at this time has a higher cobalt content, compared with sodium compounds, magnesium ions can be precipitated by lime from the final wastewater discharged, so it has less impact on the environment and tailings dam. The results of multi-element analysis of cobalt precipitation solution after de-hybridization are shown in Table 9.

Table 9 Multi-element analysis of the solution after removal of impurities
3.3.1 The effect of activated magnesium oxide plus emulsification time
Test conditions: take a certain amount of cobalt precipitation stock solution, according to the MgO/Co mass ratio of 1 to add active magnesium oxide dry powder or tap water prepared into 20% (mass concentration) emulsion to join, stirring at room temperature for 5 h after filtration, filtering, slag washing and drying sent to measure the cobalt content. The test results are shown in Table 10.

Table 10 test results of the effect of active magnesium oxide adding method
From the test results can be seen, the longer the magnesium oxide emulsification time, the worse the activity, the lower the cobalt content of the precipitated product, while the dry powder in the actual production of the addition is easy to cause agglomeration, etc., so it is recommended that magnesium oxide emulsion within 5 min to join appropriate.
3.3.2 Effect of active magnesium oxide dosage
The effect of magnesium oxide dosage on the quality and precipitation rate of cobalt hydroxide is shown in Table 11.
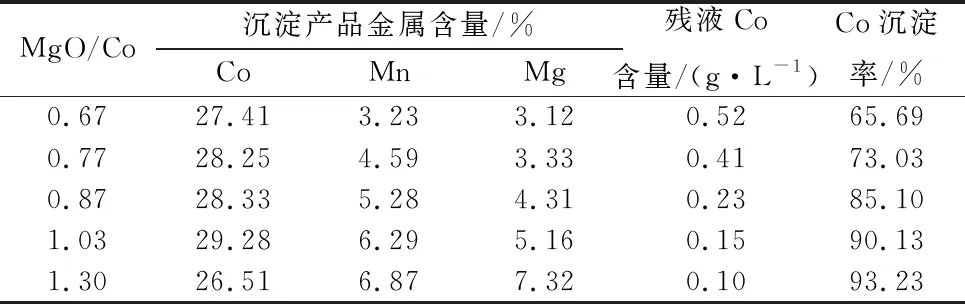
Table 11 Effect of active magnesium oxide dosage test results
Table 11 can be seen, with the increase of MgO dosage, cobalt precipitation residual liquid cobalt content gradually decreased, cobalt slag cobalt grade first increased and then decreased, when the MgO dosage and Co mass ratio of 1.03, cobalt slag with cobalt grade higher than 29% can be obtained, when the magnesium oxide is too much, cobalt hydroxide products in the manganese and magnesium content has increased dramatically, the production of the appropriate magnesium oxide dosage should be controlled.
3.3.3 The effect of cobalt sinking reaction time
Test conditions: stirring cobalt precipitation MgO/Co mass ratio of 1.03. reaction time on cobalt precipitation rate and cobalt slag quality as shown in Table 12.
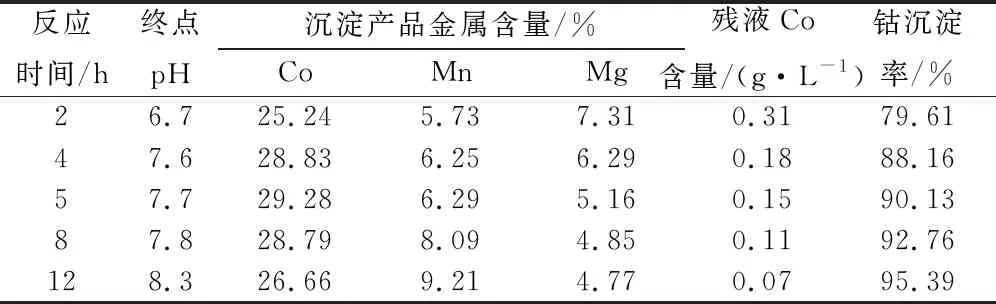
Table 12 Cobalt precipitation reaction time test results
With the prolongation of reaction time, the Co grade of cobalt precipitation slag decreases, the Mn grade increases, and the Mg grade slightly decreases. The reaction time of 4-5 h is suitable, and the cobalt precipitation rate is 90.13%.
After cobalt precipitation residual liquid also contains a certain amount of cobalt, can be used cheaper lime the second section of cobalt precipitation, the second section of cobalt precipitation slag back to the neutralization system to recover cobalt.
4.4 Comprehensive condition test of the whole process
According to the above optimization test results, the comprehensive test conditions are as follows:
(1) Copper and cobalt leaching: grinding fineness-74 μm content of 70%, stirring leaching, slurry quality concentration of 33%, adding sulfuric acid to control the endpoint pH value of 1.5 ~ 1.8, sulfuric acid added after 30 min to add sodium metabisulfite, sodium metabisulfite dosage of 8 kg / t ore, reaction time of 5h.
(2) Copper extraction residue removal: leach solution copper extraction with limestone + lime with the removal of impurities, stirring reaction at room temperature for 6 h, to control the endpoint pH in 5.8 or so.
(3) cobalt precipitation: copper extraction residue after decontamination using active magnesium oxide cobalt precipitation, active magnesium oxide emulsification within 5 min to join, MgO dosage and Co mass ratio of 1.03, cobalt precipitation time of 5 h.
The multi-element analysis results of cobalt hydroxide slag products obtained under the comprehensive test conditions are shown in Table 13 below.

Table 13 Multi-element analysis of cobalt hydroxide slag / %
The product can reach the standard of crude cobalt hydroxide second grade (“The People’s Republic of China nonferrous metal industry standard YS/T 1152-2016”).
4 Conclusion
In this study, the reduction leaching-removal of impurities-activated magnesium oxide precipitation process was developed to recover cobalt metal in a copper-cobalt ore in DRC, when the grinding fineness -74 μm content of 70%, the slurry quality concentration of 33%, adding sulfuric acid to control the endpoint pH at 1.5 ~ 1.8, sulfuric acid added for 30 min and then add sodium metabisulfite, sodium metabisulfite Sodium metabisulfite is added after 30 min of sulfuric acid addition, the dosage of sodium metabisulfite is 8 kg/t ore, leaching is stirred for 5 h, and the cobalt leaching rate is 90.38%; the leaching solution adopts oxime extractant which is mature at present to carry on the extraction – back-extraction – electrowinning, and the quality concentration of the extractant is about 20%, and the degree of sulfuric acid of back-extracting solution is about 170 g/L, and current density is about 280 A/m2, and the copper cathode products of grade A are obtained. Extracted residual solution using limestone + lime with impurity removal, stirring reaction at room temperature for 6 h, control the end point pH in 5.8 or so, lime added 3 h after the solution of copper can be reduced to 0.04 g / L, copper precipitation rate of 90.51%, iron precipitation rate of 99.23%, cobalt precipitation rate of 7.39%, precipitation slag back to the leaching process to recover copper and cobalt; removal of impurities after the solution using activated magnesium oxide cobalt precipitation, activated magnesium oxide emulsification Add within 5 min, when MgO dosage and Co mass ratio of 1.03, cobalt precipitation time of 5 h, cobalt precipitation rate of 90.13%, cobalt hydroxide precipitation in line with the industry standard requirements of second-grade products.