INTRODUCTION
Activated magnesium oxide process is a kind of hydrometallurgical technology to extract nickel and cobalt from nickel-cobalt ore with high efficiency. Using activated magnesium oxide as precipitant, this process can directly extract nickel and cobalt from nickel-cobalt ores at lower temperatures, which is characterized by low energy consumption and high output rate.

Research status

Active magnesium oxide process has a long research history in the field of nickel and cobalt hydrometallurgy. At present, the process has the following main development directions:
Raw material expansion: Explore different types of nickel and cobalt ores to expand the scope of application of the process.
Leaching optimization: Improve leaching efficiency and increase nickel and cobalt recovery.
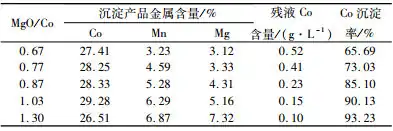
Precipitation Improvement: Optimize the precipitation conditions of magnesium oxide to improve precipitation efficiency and performance.
Tailings Utilization: Develop tailings resource utilization technology to achieve waste recycling.
Research progress
In recent years, the following major research advances have been made in the active magnesium oxide process:
Multiphase catalyst leaching: Adoption of multiphase catalyst-assisted leaching to improve nickel and cobalt leaching rate and shorten leaching time.
Pulse precipitation: Pulse precipitation technology is adopted to control the process of magnesium oxide precipitation and obtain high purity nickel and cobalt products.
Preparation of activated magnesium oxide: Different methods of preparing activated magnesium oxide were studied to improve its precipitation efficiency and stability.
Tailings Resourceization: Explore the recycling technology of iron, magnesium and other valuable metals in tailings to realize tailings resourceization.
Process Flow
The typical flow of reactive magnesium oxide process includes the following steps:
Pre-treatment of nickel and cobalt ores: crushing, grinding, etc.
Leaching: leaching nickel and cobalt at high temperature by using acidic solution or vulcanizing agent.
Solid-liquid separation: filtration or sedimentation to separate the solid-liquid phase.
Precipitation: Add active magnesium oxide solution to precipitate nickel and cobalt ions to form hydroxide.
Filtration and washing: Filter and wash the precipitate to remove impurities.
Drying: Dry the filter cake to get nickel and cobalt intermediate products or finished products.
Application prospects
Activated magnesium oxide process has the following application prospects:
Improve nickel and cobalt production capacity:The process can significantly increase the output rate of nickel and cobalt to meet the growing market demand for nickel and cobalt.
Reduce environmental pollution:The process adopts hydrometallurgical process, which greatly reduces the pollution to the environment and is conducive to promoting sustainable development.
Resource recycling: Through the tailings resourcing technology, waste recycling can be realized and resources can be saved.
Conclusion
Reactive magnesium oxide process is a highly efficient and environmentally friendly nickel and cobalt hydrometallurgical technology. The research and development of this process will play an important role in improving nickel and cobalt production capacity, reducing environmental pollution and realizing resource recycling. In the future, with the continuous improvement of the technology, the reactive magnesium oxide process is expected to become one of the key technologies for large-scale extraction of nickel and cobalt from nickel-cobalt ores.

