In citrate-soluble fertilizers, magnesium carbonate is not only a source of magnesium but also, by neutralizing acidic components and regulating pH, it can establish an effective buffering system. This enhances fertilizer stability and improves crop nutrient absorption efficiency. Therefore, it is an important and environmentally friendly choice for a multifunctional component in citrate fertilizer formulations.
1. Neutralize Excess Acidic Components
- Magnesium carbonate is an alkaline salt that reacts with acids like phosphoric acid, sulfuric acid, and nitric acid in the fertilizer to neutralize free acids.
- Reduces root irritation or soil acidification problems caused by excessive fertilizer acidity.
2. Maintain Suitable Fertilizer pH
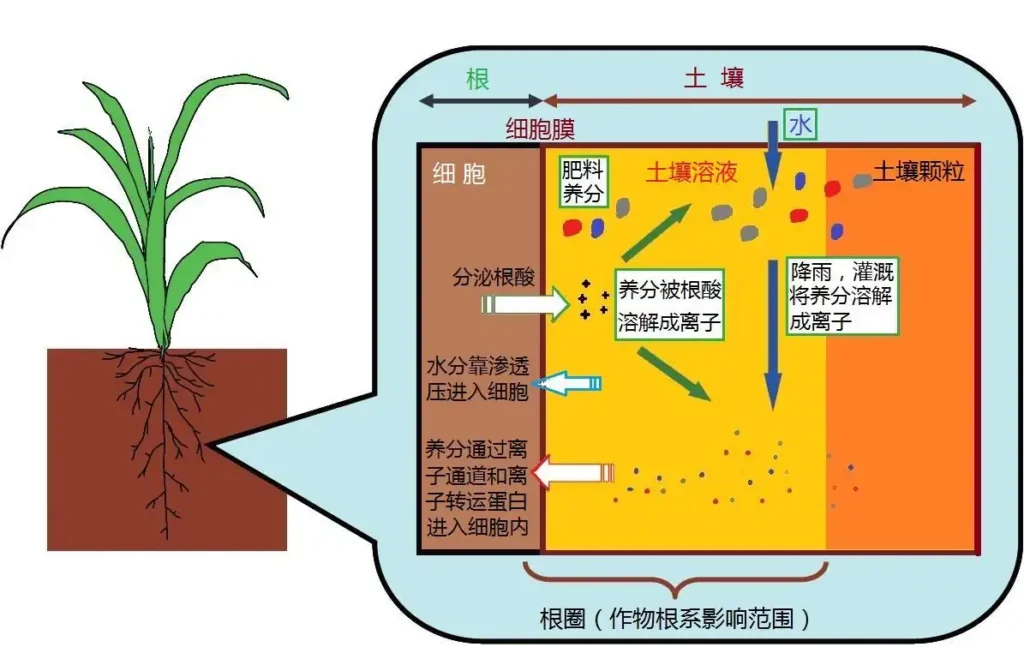
- In the production of citrate fertilizers, magnesium carbonate is used to adjust the pH to a specific range (usually 5.5–7.5).
- Ensures the dissolution stability of nutrient elements, preventing precipitation or decomposition.
3. Improve Nutrient Utilization
- A suitable fertilizer pH helps roots absorb elements like magnesium, calcium, and phosphorus.
- Avoids antagonism or fixation that renders certain elements ineffective due to acid-base imbalance.
4. Establishment of Buffering System
- Magnesium carbonate can form a buffering system in the fertilizer, slowing down pH fluctuations.
- Improves the stability of the fertilizer during storage, reducing the risk of caking or decomposition.
5. Environmental and Safety Advantages
- Magnesium carbonate is abundant, green and safe, non-toxic and harmless.
- The neutralization process is mild with low heat release, resulting in high operational safety.
